A Systematic Review of Behavioural Interventions Promoting Healthy Eating among Older People
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Screening
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis
3. Results
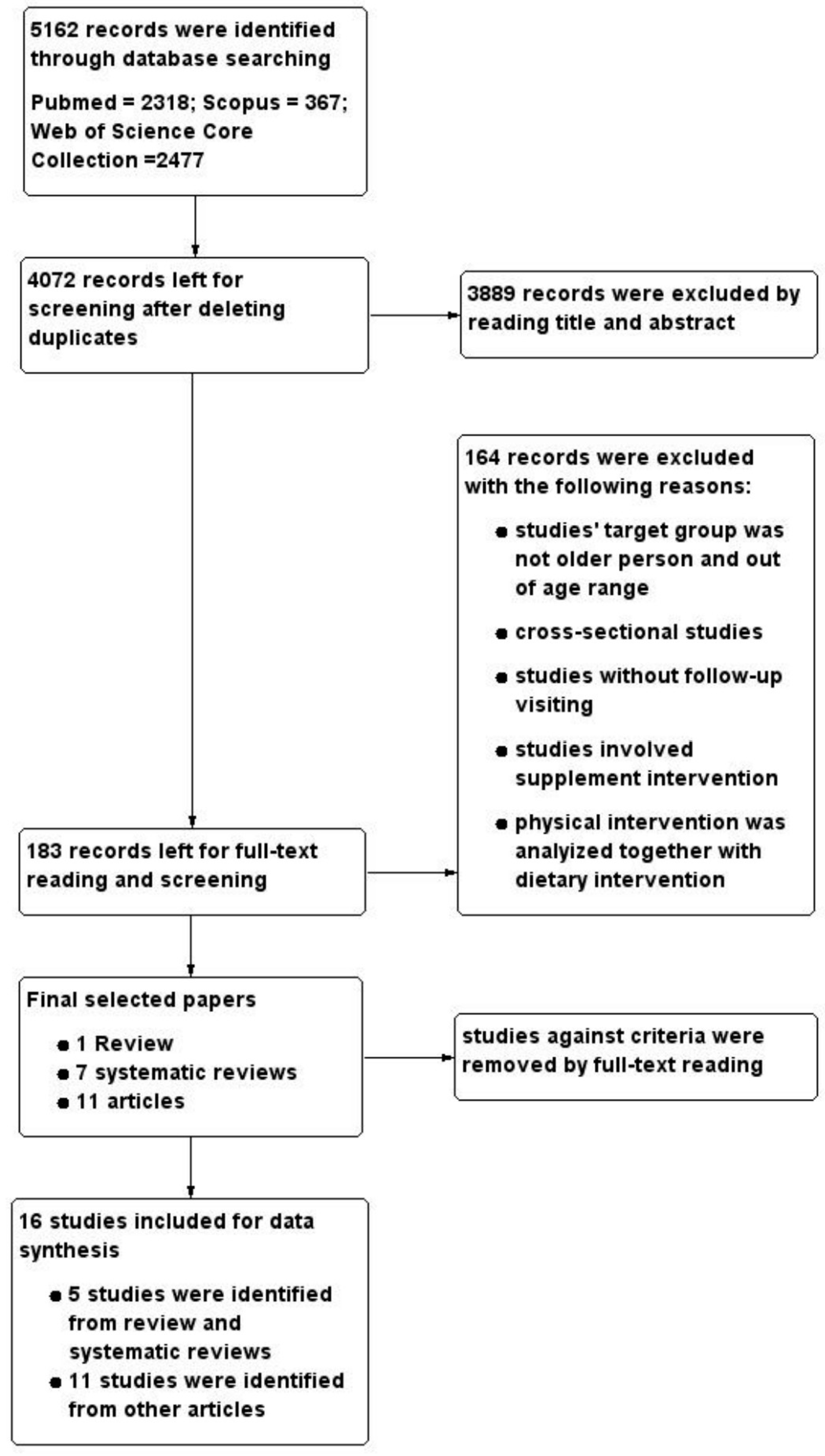
3.1. Study Selection
3.2. Study Characteristics
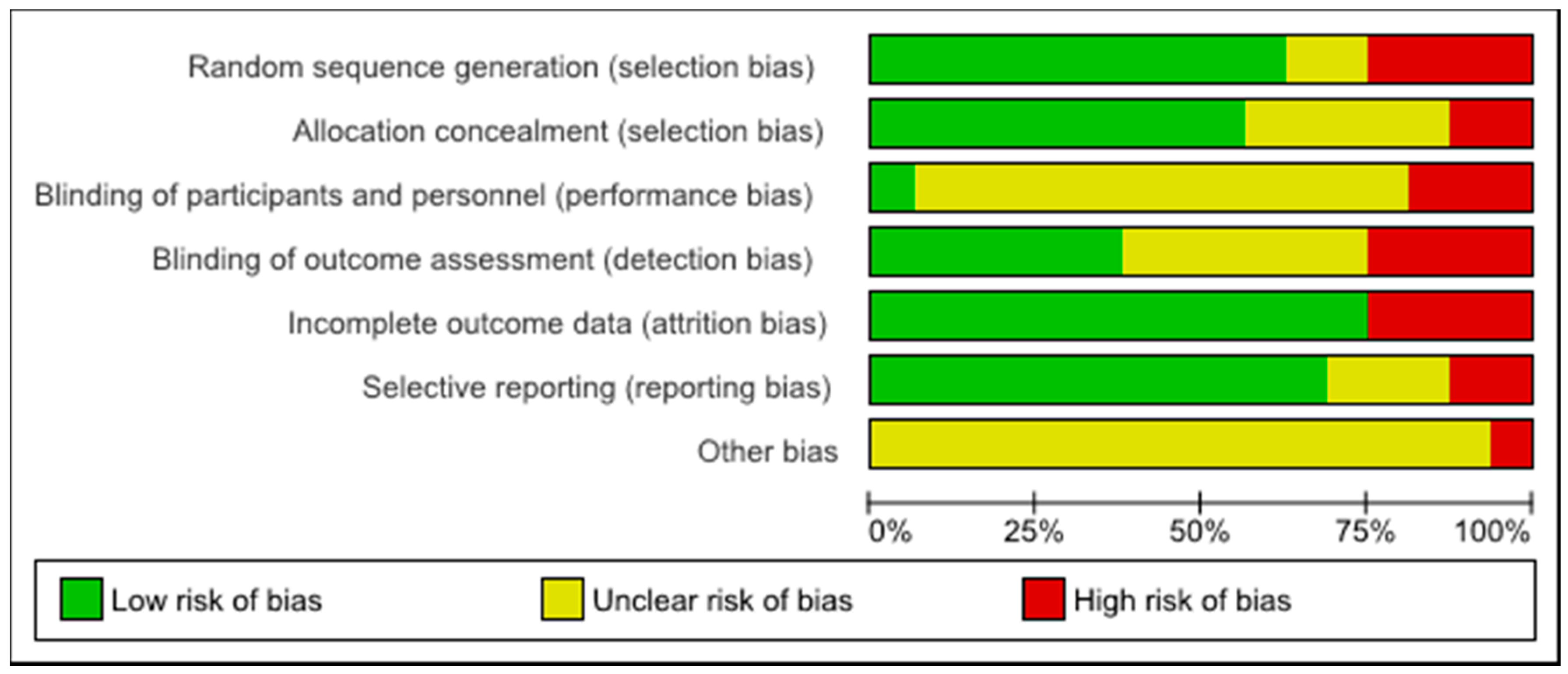
3.3. Study Quality Assessment
3.4. Effect of Interventions
3.4.1. Dietary Educational Interventions
3.4.2. Meal Service Interventions
3.4.3. Multicomponent Interventions
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2015-Highlights. Available online: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Highlights.pdf (accessed on 5 September 2016).
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Disease 2013–2020. Available online: http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf?ua=1 (accessed on 6 September 2016).
- Joyce, G.F.; Keeler, E.B.; Shang, B.; Goldman, D.P. The lifetime burden of chronic disease among the elderly. Health Aff. (Millwood) 2005, 24, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D. Malnutrition in the elderly—Prevalence, causes and corrective strategies. Clin. Nutr. 2002, 21, 110–112. [Google Scholar] [CrossRef]
- Cho, J.P.; Paek, K.W.; Song, H.J.; Jung, Y.S.; Moon, H.W. Prevalence and associated factors of falls in the elderly community. Korean J. Prev. Med. 2001, 34, 47–54. [Google Scholar]
- Saka, B.; Kaya, O.; Ozturk, G.B.; Erten, N.; Karan, M.A. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin. Nutr. 2010, 29, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Artham, S.M.; Lavie, C.J.; Patel, D.A.; Ventura, H.O. Obesity paradox in the elderly: Is fatter really fitter? Aging Health 2009, 5, 177–184. [Google Scholar] [CrossRef]
- Kvamme, J.M.; Holmen, J.; Wilsgaard, T.; Florholmen, J.; Midthjell, K.; Jacobsen, B.K.; Jacobsen, B.K. Body mass index and mortality in elderly men and women: The Tromso and HUNT studies. J. Epidemiol. Community Health 2012, 66, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Chou, Y.C.; Huang, N.; Chou, Y.J.; Hu, H.Y.; Li, C.P. Association of body mass index with all-cause and cardiovascular disease mortality in the elderly. PLoS ONE 2014, 9, e102589. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kamada, C.; Yoshimura, H.; Okumura, R.; Iimuro, S.; Ohashi, Y.; Araki, A.; Umegaki, H.; Sakurai, T.; Yoshimura, Y.; et al. Effects of total and green vegetable intakes on glycated hemoglobin A1c and triglycerides in elderly patients with type 2 diabetes mellitus: The Japanese Elderly Intervention Trial. Geriatr. Gerontol. Int. 2012, 12, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Inserra, P.F.; Watson, R.R.; Wise, J.A.; O’Neill, K.L. Supplementation with fruit and vegetable extracts may decrease DNA damage in the peripheral lymphocytes of an elderly population. Nutr. Res. 1999, 19, 1507–1518. [Google Scholar] [CrossRef]
- Toh, J.Y.; Tan, V.M.; Lim, P.C.; Lim, S.T.; Chong, M.F. Flavonoids from fruit and vegetables: A focus on cardiovascular risk factors. Curr. Atheroscler. Rep. 2013, 15, 368. [Google Scholar] [CrossRef] [PubMed]
- Inserra, P.F.; Jiang, S.; Solkoff, D.; Lee, J.; Zhang, Z.; Xu, M.; Hesslink, R.; Wise, J.; Watson, R.R. Immune function in elderly smokers and nonsmokers improves during supplementation with fruit and vegetable extracts. Integr. Med. 1999, 2, 3–10. [Google Scholar] [CrossRef]
- Lee, I.T.; Chan, Y.C.; Lin, C.W.; Lee, W.J.; Sheu, W.H.-H. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet. Med. 2008, 25, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Gerdtham, U.G.; Johansson, P. Economic evaluation of lifestyle interventions for preventing diabetes and cardiovascular diseases. Int. J. Environ. Res. Public Health 2010, 7, 3150–3195. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.; Edgar, J.; Neville, C.E.; Gilchrist, S.E.C.M.; Mckinley, M.C.; Patterson, C.C.; Young, I.S.; Woodside, J.V. Effect of fruit and vegetable consumption on immune function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.; Hemingway, A.; Saulais, L. Increasing vegetable intakes: Rationale and systematic review of published interventions. Eur. J. Nutr. 2016, 55, 869–896. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.J.F. Changes in eating behaviour during the aging process. Eat. Behav. 2002, 3, 15–43. [Google Scholar] [CrossRef]
- Mann, T.; Heuberger, R.; Wong, H. The association between chewing and swallowing difficulties and nutritional status in older adults. Aust. Dent. J. 2013, 58, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Bandayrel, K.; Wong, S. Systematic literature review of randomized control trials assessing the effectiveness of nutrition interventions in community-dwelling older adults. J. Nutr. Educ. Behav. 2011, 43, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Trabal, J.; Farran-Codina, A. Effects of dietary enrichment with conventional foods on energy and protein intake in older adults: A systematic review. Nutr. Rev. 2015, 73, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Ageing in the Twenty-First Century: A Celebration and a Challenge. Available online: https://www.unfpa.org/sites/default/files/pub-pdf/Ageing%20report.pdf (accessed on 5 September 2016).
- Sabharwal, S.; Wilson, H.; Reilly, P. Heterogeneity of the definition of elderly age in current orthopaedic research. SpringerPlus 2015, 4, 516. [Google Scholar] [CrossRef] [PubMed]
- How to Investigate the Use of Medicines by Consumers. Available online: http://www.who.int/drugresistance/Manual1_HowtoInvestigate.pdf (accessed on 8 September 2016).
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Book Series; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 187–241. ISBN 978-0-470-51845-8. [Google Scholar]
- Fernández-Real, J.M.; Bulló, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvadó, J. A Mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Lorefält, B.; Wilhelmsson, S. A multifaceted intervention model can give a lasting improvement of older peoples’ nutritional status. J. Nutr. Health Aging 2012, 16, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Mariyasu, A.; Kumagai, S.; Furuna, T.; Akita, S.; Kimura, S.; Suzuki, T. Community-based intervention to improve dietary habits and promote physical activity among older adults: A cluster randomized trial. BMC Geriatr. 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Lammes, E.; Rydwik, E.; Akner, G. Effects of nutritional intervention and physical training on energy intake, resting metabolic rate and body composition in frail elderly. A randomised, controlled pilot. J. Nutr. Health Aging 2012, 16, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gallois, K.M.; Buck, C.; Dreas, J.A.; Hassel, H.; Zeeb, H. Evaluation of an intervention using a self-regulatory counselling aid: Pre- and post-intervention results of the OPTIMAHL 60plus study. Int. J. Public Health 2013, 58, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Salehi, L.; Mohammad, K.; Montazeri, A. Fruit and vegetables intake among elderly Iranians: A theory-based interventional study using the five-a-day program. Nutr. J. 2011, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K. Increases in fruit intakes in older low consumers of fruit following two community-based repeated exposure interventions. Br. J. Nutr. 2013, 109, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunderlich, S.; Bai, Y.; Piemonte, J. Nutrition risk factors among home delivered and congregate meal participants: Need for enhancement of nutrition education and counseling among home delivered meal participants. J. Nutr. Health Aging 2011, 15, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Lorefalt, B.; Andersson, A.; Wirehn, A.B.; Wilhelmsson, S. Nutritional status and health care costs for the elderly living in municipal residential homes–An intervention study. J. Nutr. Health Aging 2011, 15, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Yates, B.C.; Pullen, C.H.; Santo, J.B.; Boeckner, L.; Hageman, P.A.; Dizona, P.J.; Walker, S.N. The influence of cognitive-perceptual variables on patterns of change over time in rural midlife and older women’s healthy eating. Soc. Sci. Med. 2012, 75, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Turbett, E.; Mckevic, A.; Rudgard, K.; Hearth, H. The Mediterranean diet among British older adults: Its understanding, acceptability and the feasibility of a randomised brief intervention with two levels of dietary advice. Maturitas 2015, 82, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kupka-Schutt, L.; Mitchell, M.E. Positive effect of a nutrition instruction model on the dietary behaviour of a selected group of elderly. J. Nutr. Elder. 1992, 12, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition 2001, 17, 709–712. [Google Scholar] [CrossRef]
- Gray-Donald, K.; Payette, H.; Boutier, V. Randomized clinical trial of nutritional supplementation shows little effect on functional status among free-living frail elderly. J. Nutr. 1995, 125, 2965–2971. [Google Scholar] [PubMed]
- Payette, H.; Boutier, V.; Coulombe, C.; Gray-Donald, K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: A prospective randomized community trial. J. Am. Diet. Assoc. 2002, 102, 1088–1095. [Google Scholar] [CrossRef]
- McNeill, G.; Avenell, A.; Campbell, M.K.; Cook, J.A.; Hannaford, P.C.; Kilonzo, M.M.; Milne, A.C.; Ramsay, C.R.; Seymour, D.G.; Stephen, A.I.; et al. Effect of multivitamin and multi-mineral supplementation on cognitive function in men and women aged 65 years and over: A randomized con-trolled trial. Nutr. J. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; An, R. Impact of home-delivered meal programs on diet and nutrition among older adults. Nutr. Health 2013, 22, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Troyer, J.L.; Racine, E.F.; Ngugi, G.W.; McAuley, W.J. The effect of home-delivered dietary approach to stop hypertension (DASH) meals on the diets of older adults with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Racine, E.F.; Lyerly, J.; Troyer, J.L.; Warren-Findlow, J.; McAuley, W.J. The influence of home-delivered dietary approaches to stop hypertension meals on body mass index, energy intake, and percent of energy needs consumed among older adults with hypertension and/or hyperlipidemia. J. Acad. Nutr. Diet. 2012, 112, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.F.; Bryan, J.D. Dietary intake of homebound elderly recipients and nonrecipients of home-delivered meals. J. Nutr. Elder. 1986, 5, 23–34. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.M.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cueto, F.J.; Aschemann-Witzel, J.; Shankar, B.; Brambila-Macias, J.; Bech-Larsen, T.; Mazzocchi, M.; Capacci, S.; Saba, A.; Turrini, A.; Niedzwiedzka, B.; et al. Assessment of evaluations made to healthy eating policies in Europe: A review within the EATWELL Project. Public Health Nutr. 2012, 15, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Bucher, T.; Collins, C.; Rollo, M.E.; McCaffrey, T.A.; De Vlieger, N.; Van der Bend, D.; Truby, H.; Perez-Cueto, F.J.A. Nudging consumers towards healthier choices: A systematic review of positional influences on food choice. Br. J. Nutr. 2016, 115, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.; Hershey, M.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Perälä, M.-M.; von Bonsdorff, M.; Männistö, S.; Salonen, M.K.; Simonen, M.; Kanerva, N.; Pohjolainen, P.; Kajantie, E.; Rantanen, T.; Eriksson, J.G. A healthy Nordic diet and physical performance in old age: Findings from the longitudinal Helsinki Birth cohort study. Br. J. Nutr. 2016, 115, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Lacoppidan, S.; Kyrø, C.; Loft, S.; Helnæs, A.; Christensen, J.; Hansen, C.; Dahm, C.; Overvad, K.; Tjønneland, A.; Olsen, A. Adherence to a healthy Nordic food index is associated with a lower risk of type-2 diabetes—The Danish diet, cancer and health cohort study. Nutrients 2015, 7, 8633–8644. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet quality as assessed by the Healthy Eating Index, alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: An updated systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2018, 118, 74–100. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.; Whear, R.; Thompson-Coon, J. Effectiveness of mealtime interventions on nutritional outcomes for the elderly living in residential care: A systematic review and meta-analysis. Ageing Res. 2013, 12, 967–981. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.H.; Hunt, A.; Hackes, B.; Pope, J.F. Impact of dining room environment on nutritional intake of Alzheimer’s residents: A case study. Am. J. Alzheimer’s Dis. Other Dement. 2001, 16, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Yale University Press: New Haven, CT, USA, 2008; pp. 1–6. ISBN 978-0-300-12223-7. [Google Scholar]
- Hansen, P.G.; Skov, L.R.; Skov, K.L. Making healthy choices easier: Regulation versus nudging. Annu. Rev. Public Health 2016, 37, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Skov, L.R.; Lourenço, S.; Hansen, G.L.; Mikkelsen, B.E.; Schofield, C. Choice architecture as a means to change eating behaviour in self-service settings: A systematic review. Obes. Rev. 2013, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]

| Study | Country | Age (Years) | Setting | Sample Size | Study Design | Description of Intervention | Comparison | Duration | Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|
| Fernández-Real 2012 [31] | Spain | 55–80 1 | PREDIMED study centre | 127 | RCT | Participants were randomly assigned to the MD + EVOO and MD + NUTS group; dietitians gave personalized dietary advice to participants corresponding to different diets | Control group (low-fat diet) | 2 years | Total osteocalcin; procollagen 1 N-terminal propeptide levels; homeostasis model assessment-β-cell function. |
| Lorefält 2012 [32] | Sweden | 83.8 ± 7.7 2 | Residential homes | 67 | Within-subjects design 3 | A multifaceted intervention model including education on both theoretical and practical issues for staff; individualized snacks were served to the residents | Participants were their own controls | 1 year | Energy intake; Body weight; MNA score; length of night-time fasting |
| Kimura 2013 [33] | Japan | 65–90 1 | Community centre | Baseline: 141 Intervention: 92 | Cluster-RCT | Consisted of a general lecture on the importance of dietary variety and five educational sessions. | The control group was subsequently provided with the same program as a crossover intervention group | 3 months | Food intake; frequency score; dietary variety score; self-rated health; appetite; TMIG Index of Competence |
| Gibson 2012 [21] | UK | 65–85 1 | Residential area | 82 | RCT | Intervention group: FV intake ≥5 portions/day | Normal diet (FV intake ≤2 portions/day) | 16 weeks | Changes of FV intake (Mean ± SD); antibody assessment |
| Lammes 2012 [34] | Sweden | ≥75 1 | Elderly research centre | Baseline: 95 Intervention: 79 follow-up: 64 | RCT (Pilot study) | Three types of intervention (1) Nutritional intervention: individual dietary counselling and estimation of each participant’s energy needs (2) Physical training (3) Combined nutritional and physical intervention | General advice regarding diet and physical training | 1 year | Energy intake; resting metabolic rate; fat-free mass |
| Gallois 2013 [35] | Germany | ≥57 1 | Low socio-economic status district: community partners’ institution, churches and mosques | Baseline: 423 Intervention: 369 | Quasi- Experimental Study | The intervention comprised seven sessions. In each session, older participants discussed health topics and received counselling aid; standard health information on physical activity and nutrition, and cooking recipes were handed out at the end of each session | The control group only received standard health information and cooking recipes by post | 1 year | Changes of FV intake; dairy product and fish intake (Mean ± SD) |
| Salehi 2011 [36] | Iran | 64.06 ± 4.48 2 | Elderly centre | Intervention group: 200 Control group: 200 | Quasi-Experimental Study | Participants received four weekly sessions including introduction, stages of change for FV intake, reinforcement of second session, and barriers anticipated and overcome | Control group: general health education | 4 weeks | Changes in food intake (mean serving/day); stage transitions; self-efficacy; perceived benefits and barriers |
| Appleton 2013 [37] | UK | ≥65 1 | Community-based church and social group | 95 | Quasi-RCT | Participants were randomized to receive five (n = 38) or five plus (n = 18) exposures of fruit over a 5-week period | One-time exposure | 5 weeks | Fruit intake and liking; FV intake and liking (Mean ± SD) |
| Sánchez-Villegas 2013 [38] | Spain | Men: 55–80 women: 60–80 1 | Primary care centre | MD + EVOO: 1446 MD + NUTS: 1293 Control group: 1184 | RCT | Participants were randomly assigned to the MD + EVOO and MD + NUTS group, and received intensive education on MD | Low-fat diet including recommendations to reduce all types of fat intake | 3 years | Risk of incidence of depression |
| Wunderlich 2011 [39] | USA | ≥60 1 | Congregate and home delivered meal locations | Baseline: 476 Intervention: 355 | Quasi-Experimental Study | CGM (congregate meal) participants: regular topical nutrition education and counselling in a classroom format with cooking demo, discussion, and handouts | The HDM (home delivered meal): participants only received the printed material (same handouts) and counselling by telephone | 2 years | FV intake (%); Nutrition risk score; Meal intake/day |
| Lorefält 2011 [40] | Sweden | 83–86 1 | Residential homes | Intervention group: 42 Control group: 67 | Quasi-Experimental Study | A multifaceted intervention design was adopted; nutritional status of older participants was measured by MNA; individualized meals were provided to the residents based on the results of the MNA | Only received education on how to measure MNA; residents from the control group followed the usual meal routines | 3 months | Body weight; MNA score; cost of health care |
| Salas-Salvadó 2014 [41] | Spain | Men: 55–80 women: 60–80 1 | Primary care centre | MD + EVOO: Intervention: 2543 (follow up: 1154) MD + NUTS: Intervention: 2454 (follow up: 1240) Control group: 2450 (follow up: 1147) | RCT | Participants were randomly assigned to the MD + EVOO and MD + NUTS group; dietitians conducted individual and group dietary training sessions to provide information on typical Mediterranean foods, seasonal shopping lists, meal plans, and recipes | Received only a leaflet describing low-fat diet | 7 years | Incidence of diabetes; MD adherence; MD score 4 |
| Estruch 2013 [42] | Spain | Men: 55–80 women: 60–80 1 | PREDIMED study centre | MD + EVOO: 2543 MD + NUTS: 2454 Control group: 2450 | RCT | Participants were randomly assigned to the MD + EVOO and MD + NUTS group; dietitians ran individual and group dietary-training sessions at the baseline visit and quarterly thereafter | Control group received small non-food gifts | 4.8 years | Rate of cardiovascular events |
| Salas-Salvadó 2011 [20] | Spain | Men: 55–80 women: 60–80 1 | PREDIMED study centre | 418 | RCT | Participants were randomly assigned to the MD + EVOO and MD + NUTS group; dietitians gave personalized dietary advice to participants | Received only a leaflet describing the low-fat diet | 5 years | Incidence of diabetes |
| Yates 2012 [43] | USA | Women: 50–69 1 | Rural research offices | 225 | Cluster-RCT | A repeated-measures experimental design: intervention group received tailored newsletter | Group received standard newsletter | 2 years | Self-efficacy; benefits of healthy eating; family support; perceived barriers |
| Lara 2015 [44] | UK | ≥50 1 | Human nutrition research centre | 23 | RCT | Evaluated the feasibility of a three-week brief MD intervention with two levels of dietary advice; Level 2: EGS and received additional support | Level 1: only attended an EGS | 3 weeks | Food intake (Mean ± SD); MD score; cost of adopting an MD/day |
| Study | Main Outcomes |
|---|---|
| Dietary educational interventions | |
| Kimura 2013 [33] | Percentage of participants who scored 1–3 regarding the dietary variety showed a significant difference (p = 0.041) between the intervention group and control group. Improvement rate of self-rated health did not show a significant difference between the control group and intervention group. Compared with the baseline, there was a significant increase of post-intervention food intake frequency in the intervention group in the following items: daily consumption of meat +19.3%, p = 0.002; fish/shellfish +8.7%, p = 0.02; eggs +8.8%, p = 0.01; potatoes + 10.5%, p = 0.019; fruits +10.5%, p = 0.029; seaweed +22.8%, p = 0.001; an increase in food frequency score (mean +2.4 points, p < 0.001); in dietary variety score (mean +1.2 units, p = 0.001); in self-rated health (7% were in ‘not good’ category, p = 0.003). In the control group, there was no significant difference between the baseline and post-intervention. Appetite and TMIG Index of Competence score did not change between baseline and post-intervention in both groups. |
| Lammes 2012 [34] | Individual nutrition counselling had no effect on energy intake, resting metabolic rate, and fat-free mass. |
| Gallois 2013 [35] | No significant differences were found between the control group and intervention group at the first follow-up. Compared with the baseline, except for dairy product consumption, there were significant increases of daily fruit and vegetable consumption (+23 participants reached the recommended level, p = 0.04) and weekly fish consumption (+33 participants reached the recommended level, p = 0.04) in the intervention group at the first follow-up. Similar results were shown in the control group. Dairy product consumption did not present any changes. |
| Salehi 2011 [36] | Compared with the control group, the intervention group showed significant increase of FV intake (mean +1.3 servings/day, p = 0.001), perceived benefits (mean +9.44 points, p < 0.001) and self-efficacy (mean +5.64 points, p < 0.001), but lower perceived barriers (mean −6.9% points, p < 0.001) at post-test assessment. Compared with the control group, a larger percentage of older people in the intervention group moved from precontemplation to contemplation/preparation and action/maintenance stages (p < 0.0001), and from contemplation/preparation to action/maintenance stages (p = 0.004). |
| Wunderlich 2011 [39] | Nutrition education and counselling improved nutrition risk scores significantly in HDM group (mean −2 points, p < 0.01) but not in CGM group (mean −0.44 points, p = 0.14). Slight improvements in nutrition behaviours were found in HDM group eating ≥2 meals (+5.6%) and CGM group eating ≥5 servings of fruits and vegetables (+3.4%). |
| Yates 2012 [43] | Self-efficacy and benefits of healthy eating did not change significantly over time between groups (tailored newsletter group and standard group). At the end of intervention, the tailored newsletter group got significantly more family support (b = −0.289, β = −0.366, z = 2.4, p < 0.05) and a less perceived barrier than the standard group (b = 0.14, β = 0.369, z = 2.42, p < 0.05). |
| Lara 2015 [44] | No significant differences were shown in group 1 (educational group session on MD) and group 2 (educational group session on MD with additional support). Compared with the baseline, mean fish intake (+25.9, p = 0.01) and mean MD score (+0.6 points, p = 0.05) increased significantly when analysing the combined group, but no significant difference was found in food intake cost. |
| Meal service interventions | |
| Lorefält 2012 [32] | MNA score significantly increased after 3 months’ intervention (mean +1.3 points, p = 0.01) and it was maintained after 9 months; weight (mean +1.9 kg, p = 0.0001) and energy intake (me-an +376 kcal, p = 0.0001) increased significantly during the whole period; length of night-time fasting decreased (after 3 months’ intervention: −0.8 h, p = 0.0001, after 9 months’ intervention: −0.6 h, p = 0.01), but not to the recommended level. |
| Gibson 2012 [21] | After 16 weeks, the change in FV intake showed a significant difference (p < 0.001) between 2-portion/day group (0.4 portions/day) and 5-portion/day group (4.6 portions/day); antibody binding to pneumococcal capsular polysaccharide increased more in the 5-portion/day group than in the 2-portion/day group (geometric mean +1.4, p = 0.005). |
| Appleton 2013 [37] | At week 1, except for liking familiar fruits, no differences were found in other measures between any groups. In low fruit intake consumers, a significant increase of fruit intake was found in the repeated groups (five or five plus exposures to fruit: mean 0.6 and 0.8 portions/day, respectively, p < 0.01), but not in the one-time fruit exposure group (mean 0.3 portions/day, p = 0.78) at week 1.Similar results were found over the whole experiment duration. No differences were found between the five and five plus exposure groups (p = 0.31). No changes in liking were identified over time or between repeated exposure groups, but familiar fruits showed an increase in liking (p < 0.01) than novel fruit products and dishes. Similar exposure effects were also shown on FV intake and liking (FV intake increase in the repeated exposure group: p = 0.01, in one-time fruit exposure group: p = 0.97). |
| Lorefält 2011 [40] | After 3 months, MNA score (malnourished −14.3%, p < 0.01) and body weight (mean +2.7 kg, p < 0.001) increased significantly in the intervention group compared with the control group; cost of primary health care occupied about 80% of the total median cost in the intervention group and about 55% in the control group. |
| Multicomponent Interventions | |
| Fernández-Real 2012 [31] | The total osteocalcin (mean +1.5 ng/mL, p = 0.007), procollagen 1 N-terminal propeptide levels (mean +71.6 ng/mL, p = 0.01) and homeostasis model assessment-β-cell function (mean +11.5 units, p = 0.01) increased significantly in MD +EVOO group, but not in the MD +NUTS group (p = 0.32) and control groups (p = 0.74) after the intervention period. |
| Sánchez-Villegas 2013 [38] | Risk of depression in participants assigned to MD + NUTS was inversely associated with the control group, but not significant. When analysis targeted participants with type 2 diabetes, risk of depression showed significant reduction in participants assigned to MD + NUTS compared with the control group (−41%, p = 0.04). |
| Salas-Salvadó 2014 [41] | During follow-up, mean scores of adherence to the Mediterranean diet increased in the Mediterranean diet group compared with the control group (mean +around 1.5–2 points, p < 0.01); proportion of participants with a Mediterranean diet score of 10 or higher was larger in the Mediterranean diet group than in the control group (p < 0.010) over the whole duration. Rates of diabetes cases in MD + EVOO group, MD + NUTS group and control group were 16.0, 18.7, and 23.6 cases per 1000 person years, respectively. Multivariate-adjusted hazard ratios for MD + EVOO group and MD + NUTS group were 0.60 and 0.82 compared with the control group. When considering the two MD groups together, diabetes incidence was reduced (−30%) compared with the control group. |
| Estruch 2013 [42] | Compared with the control group, multivariate-adjusted hazard ratios in MD + EVOO group and MD + NUTS group were 0.70 and 0.72, respectively. Cardiovascular risk was reduced (around 30%) by MD + EVOO or MD + NUTS. |
| Salas-Salvadó 2011 [20] | Diabetes incidence in MD + EVOO group, MD + NUTS group, and control group were 10.1%, 11.0%, and 17.9%, respectively. When considering the two MD groups together, diabetes incidence reduced (−52%) when compared with the control group. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Perez-Cueto, F.J.A.; Santos, Q.D.; Monteleone, E.; Giboreau, A.; Appleton, K.M.; Bjørner, T.; Bredie, W.L.P.; Hartwell, H. A Systematic Review of Behavioural Interventions Promoting Healthy Eating among Older People. Nutrients 2018, 10, 128. https://doi.org/10.3390/nu10020128
Zhou X, Perez-Cueto FJA, Santos QD, Monteleone E, Giboreau A, Appleton KM, Bjørner T, Bredie WLP, Hartwell H. A Systematic Review of Behavioural Interventions Promoting Healthy Eating among Older People. Nutrients. 2018; 10(2):128. https://doi.org/10.3390/nu10020128
Chicago/Turabian StyleZhou, Xiao, Federico J. A. Perez-Cueto, Quenia Dos Santos, Erminio Monteleone, Agnès Giboreau, Katherine M. Appleton, Thomas Bjørner, Wender L. P. Bredie, and Heather Hartwell. 2018. "A Systematic Review of Behavioural Interventions Promoting Healthy Eating among Older People" Nutrients 10, no. 2: 128. https://doi.org/10.3390/nu10020128






