Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Cardiometabolic, Anthropometric and Blood Pressure Measurements
2.4. Demographic Characteristics
2.5. MEDAS
2.6. Reference Instrument
2.7. Statistical Analyses
2.7.1. Concurrent Validity
2.7.2. Predictive Validity
2.7.3. Test-Retest Reliability
3. Results
3.1. Participant Characteristics
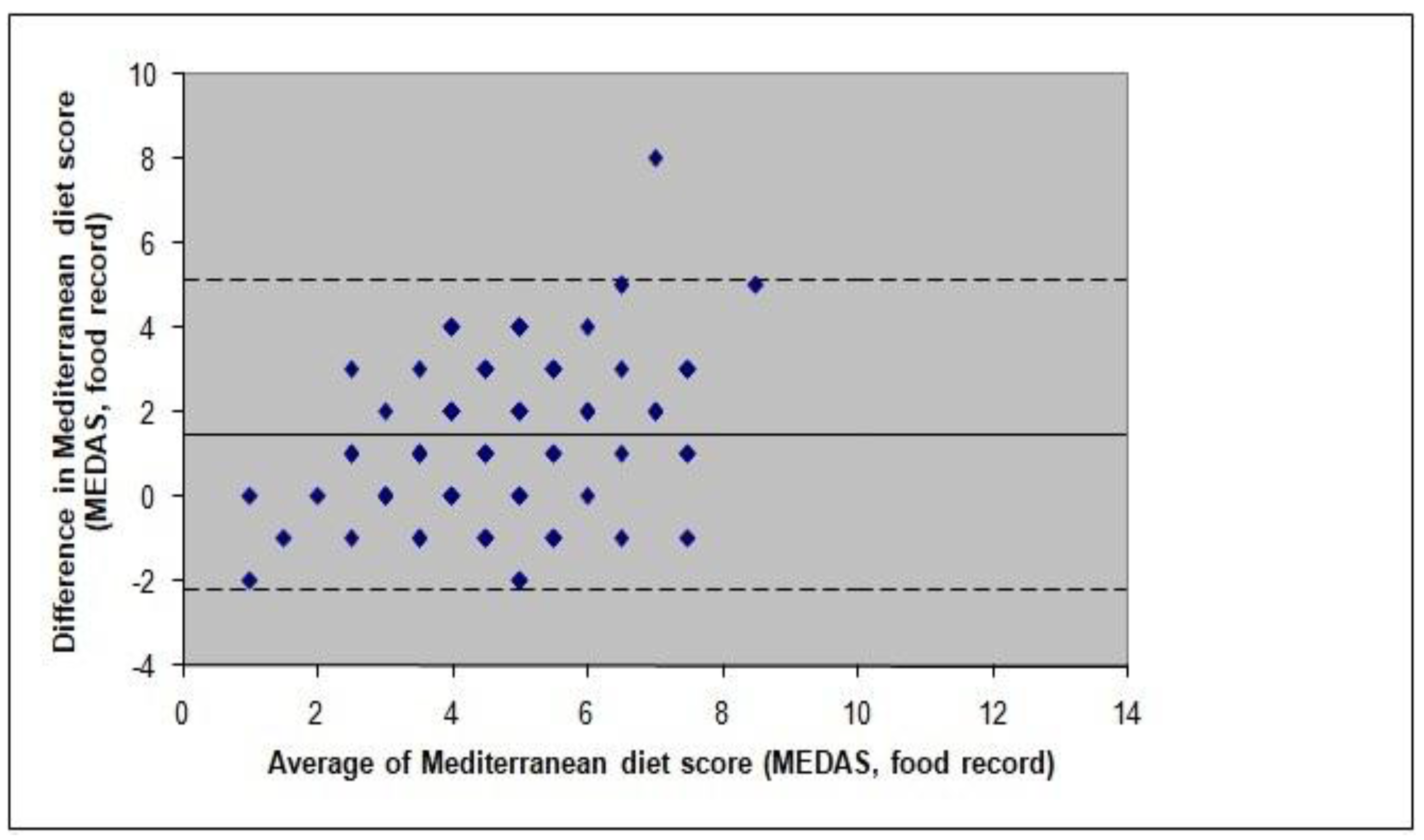
3.2. Concurrent Validity
3.3. Predictive Validity
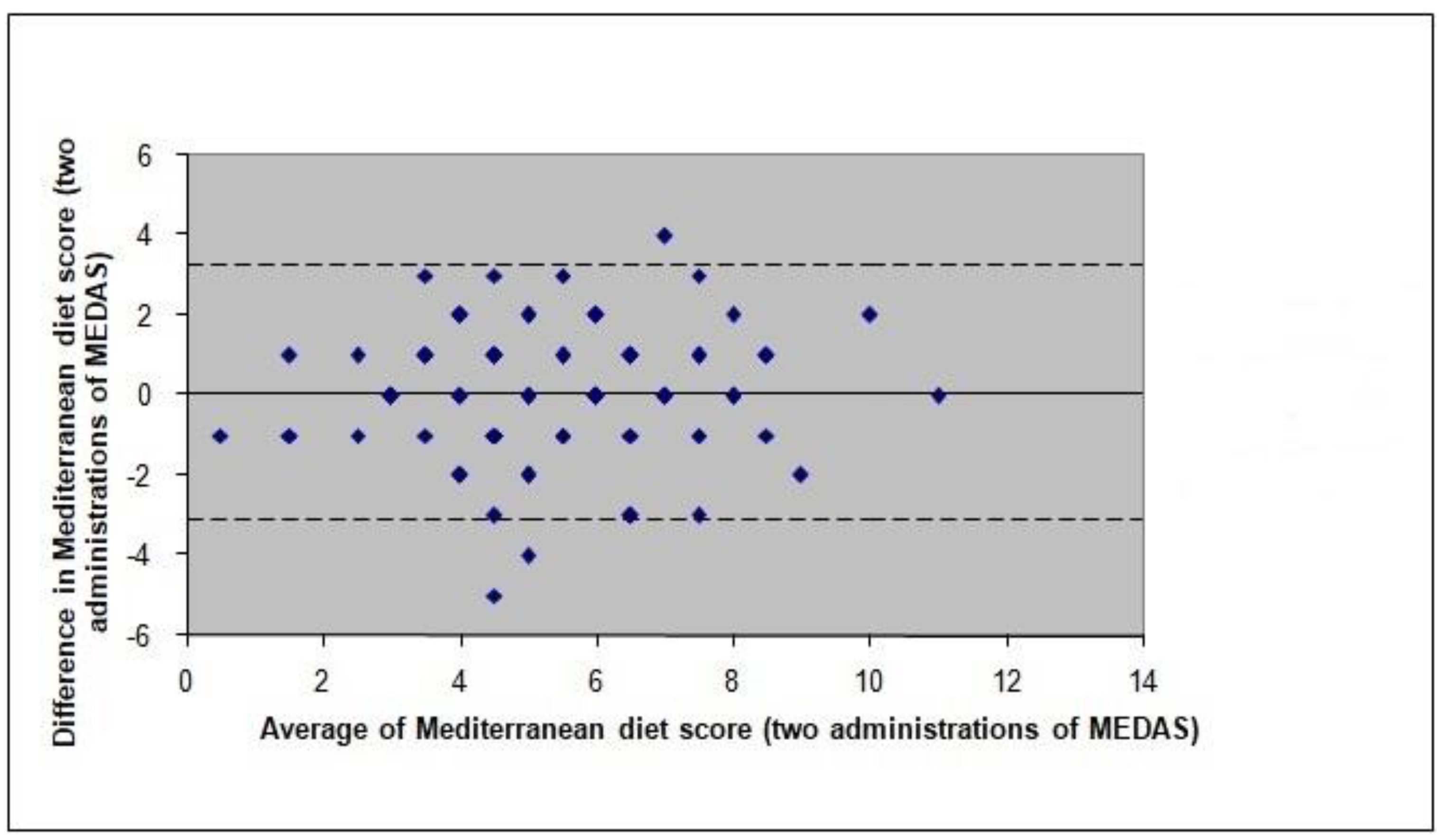
3.4. Test-Retest Reliability
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anand, S.S.; Yusuf, S. Stemming the global tsunami of cardiovascular disease. Lancet 2011, 377, 529–532. [Google Scholar] [CrossRef]
- Townsend, N.; Williams, J.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M. Cardiovascular Disease Statistics, 2014; British Heart Foundation: London, UK, 2014. [Google Scholar]
- Health and Social Care Information Centre. National Diabetes Audit 2011/12: Report 2: Complications and Mortality. Available online: https://digital.nhs.uk/search?q=National+Diabetes+Audit+2011%2F12%3A+Report+2%3A+Complications+and+Mortality&s=s (accessed on 5 November 2017).
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001, 44 (Suppl. S2), 14–21. [Google Scholar] [CrossRef]
- Keys, A. Coronary heart disease in seven countries. Circulation 1970, 41 (Suppl. S1), 1–211. [Google Scholar] [CrossRef]
- Willett, W. The Mediterranean diet: Science and practice. Public Health Nutr. 2006, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruiz-Gutiérrez, V.; Lamuela-Raventos, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union. Council Conclusions on Nutrition and Physical Activity. Available online: https://epha.org/council-conclusions-on-nutrition-and-physical-activity/ (accessed on 5 November 2017).
- Lairon, D. Intervention studies on Mediterranean diet and cardiovascular risk. Mol. Nutr. Food Res. 2007, 51, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Papadaki, A.; Linardakis, M. The use of composite scores to assess adherence to dietary patterns: The Mediterranean diet case. In Appetite and Nutritional Assessment, Nutrition and Diet Research Progress Series, 1st ed.; Ellsworth, S.J., Schuster, R.C., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 285–354. [Google Scholar]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Hebestreit, K.; Yahiaoui-Doktor, M.; Engel, C.; Vetter, W.; Siniatchkin, M.; Erickson, N.; Halle, M.; Kiechle, M.; Bischoff, S.C. Validation of the German version of the Mediterranean Diet Adherence Screener (MEDAS) questionnaire. BMC Cancer 2017, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Entwistle, T.R.; Fildes, J.E.; Green, A.C. Relative validity of short questionnaires to assess Mediterranean diet or low-fat diet adherence. J. Aging Res. Clin. Pract. 2017, 6, 23–27. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Altman, D.G. Predicting the 10 year risk of cardiovascular disease in the United Kingdom: Independent and external validation of an updated version of QRISK2. Br. Med. J. 2012, 344, e4181. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Office for National Statistics. Ethnicity and National Identity in England and Wales 2011. Available online: http://www.ons.gov.uk/ons/dcp171776_290558.pdf (accessed on 16 June 2014).
- Grundy, S.; Jamieson, L. UK Sociodemographic Profile of 18–24 Year Olds. Available online: http://www.sociology.ed.ac.uk/youth/docs/UK_sociodem.pdf (accessed on 16 June 2014).
- Schneider, S. Measuring Educational Attainment. Available online: https://www.ukdataservice.ac.uk/media/262853/discover_sqb_education_schneider.pdf (accessed on 16 June 2014).
- Nelson, M.; Atkinson, M.; Meyer, J. A Photographic Atlas of Food Portion Sizes; Ministry of Agriculture, Fisheries and Food: London, UK, 1997. [Google Scholar]
- Public Health England. National Diet and Nutrition Survey: Results from Years 1–4 (Combined) of the Rolling Programme (2008/2009–2011/12): Executive Summary. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/310997/NDNS_Y1_to_4_UK_report_Executive_summary.pdf (accessed on 5 November 2017).
- Food Standards Agency. McCance and Widdowson’s the Composition of Foods, 6th ed.; Royal Society of Chemistry: Cambridge, UK, 2002.
- Altman, D. Practical Statistics for Medical Research; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Altman, D.; Bland, J. Quartiles, quintiles, centiles, and other quantiles. Br. Med. J. 1994, 309, 996. [Google Scholar] [CrossRef]
- Bland, J.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 8, 307–310. [Google Scholar] [CrossRef]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; John Wiley Sons: New York, NY, USA, 1986. [Google Scholar]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; O’Reilly, L.M.; Whybrow, S.; Fuller, Z.; Johnstone, A.M.; Livingstone, M.B.E.; Ritz, P.; Horgan, G.W. Measuring the difference between actual and reported food intakes in the context of energy balance under laboratory conditions. Br. J. Nutr. 2014, 111, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.C.; Westerterp, K.R. Improved reporting of habitual food intake after confrontation with earlier results on food reporting. Br. J. Nutr. 2007, 83, 363–369. [Google Scholar]
- Kirkpatrick, S.I.; Midthune, D.; Dodd, K.W.; Potischman, N.; Subar, A.F.; Thompson, F.E. Reactivity and its association with body mass index across days on food checklists. J. Acad. Nutr. Diet. 2012, 112, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, A.; Verhagen, H.; Moschandreas, J.; Apostolaki, I.; Van Westerop, J. Mediterranean diet of Crete: Foods and nutrient content. J. Am. Diet. Assoc. 2000, 100, 1487–1493. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kouris-Blazos, A.; Vassilakou, T.; Gnardellis, C.; Polychronopoulos, E.; Venizelos, M.; Lagiou, P.; Wahlqvist, M.; Trichopoulos, D. Diet and survival of elderly Greeks: A link to the past. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1346S–1350S. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Shook, J.; Kerstetter, J.; Kenny, A.; Bihuniak, J.; Huedo-Medina, T. The efficacy of the Mediterranean diet on obesity outcomes: A meta-analysis. FASEB J. 2015, 29, 254. [Google Scholar]
- Garcia, M.; Bihuniak, J.D.; Shook, J.; Kenny, A.; Kerstetter, J.; Huedo-Medina, T.B. The effect of the traditional Mediterranean-style diet on metabolic risk factors: A meta-analysis. Nutrients 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Sanchez-Tainta, A.; Estruch, R.; Lamuela-Raventos, R.M.; Schröder, H.; Salas-Salvadó, J.; Corella, D.; Fiol, M.; Gomez-Gracia, E.; Arós, F.; et al. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: The PREDIMED study. J. Am. Diet. Assoc. 2008, 108, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Turconi, G.; Celsa, M.; Rezzani, C.; Biino, G.; Sartirana, M.A.; Roggi, C. Reliability of a dietary questionnaire on food habits, eating behaviour and nutritional knowledge of adolescents. Eur. J. Clin. Nutr. 2003, 57, 753–763. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | n | % |
|---|---|---|
| Sex | ||
| Males | 34 | 34.3 |
| Females | 65 | 65.7 |
| Ethnic background | ||
| Caucasian | 92 | 92.9 |
| Other | 7 | 7.1 |
| Marital status | ||
| Single | 14 | 14.1 |
| Married/cohabiting | 54 | 54.6 |
| Divorced/separated/widowed | 31 | 31.3 |
| Education | ||
| No qualifications | 31 | 31.3 |
| GCE ‘O’ levels, CSE, GCSE | 21 | 21.2 |
| GCE ‘A’ level or equivalent | 11 | 11.1 |
| Further education (e.g., HNC, HND) | 4 | 4.1 |
| Degree or equivalent | 21 | 21.2 |
| Postgraduate degree | 11 | 11.1 |
| Occupation | ||
| Retired | 75 | 75.8 |
| Employed | 23 | 23.2 |
| Unemployed | 1 | 1 |
| Current smokers 1 | 15 | 15.1 |
| Body weight status | ||
| Underweight | 2 | 2 |
| Normal weight | 21 | 21.4 |
| Overweight | 42 | 42.9 |
| Obese | 33 | 33.7 |
| Age, years 2 | 68.3 ± 6.0 (56–80) | |
| Body mass index, kg/m2 (n = 98) 3 | 28.3 ± 4.4 | |
| Waist circumference, cm (n = 99) 3 | 99.3 ± 11.9 | |
| Systolic blood pressure, mmHg (n = 95) 3 | 133.8 ±17.1 | |
| Diastolic blood pressure, mmHg (n = 95) 3 | 79.5 ± 9.5 | |
| Total cholesterol, mmol/L (n = 87) 3 | 5.5 ± 1.2 | |
| LDL-cholesterol, mmol/L (n = 86) 3 | 3.2 ± 1.3 | |
| HDL-cholesterol, mmol/L (n = 87) 3 | 1.6 ± 0.5 | |
| Non-HDL-cholesterol, mmol/L (n = 87) 3 | 3.9 ± 1.1 | |
| Triglycerides, mmol/L (n = 87) 3 | 1.7 ± 0.9 | |
| Total:HDL-cholesterol ratio (n = 87) 3 | 3.7 ± 1.2 | |
| Triglyceride:HDL-cholesterol ratio (n = 87) 3 | 1.2 ± 0.9 | |
| Glycosylated haemoglobin (HbA1c), % (n = 54) 3 | 6.0± 3.1 | |
| MEDAS | 3-Day Food Record | κ (95% CIs) | p-Value | |
|---|---|---|---|---|
| Olive oil for cooking | 50 (52.1) | 38 (39.6) | 0.09 (−0.12, 0.28) | 0.356 |
| Total olive oil consumed | 1 (1.0) | 1 (1.0) | −0.01 (−0.03, 0.00) | 0.918 |
| Vegetables | 10 (10.4) | 5 (5.2) | 0.21 (−0.07, 0.52) | 0.026 |
| Fruit | 35 (36.5) | 24 (25.0) | 0.49 (0.29, 0.66) | <0.001 |
| Red and processed meat | 75 (78.1) | 69 (71.9) | 0.34 (0.11, 0.54) | 0.001 |
| Butter, margarine, cream | 58 (60.4) | 51 (53.1) | 0.18 (−0.02, 0.37) | 0.080 |
| Sugar sweetened beverages | 69 (71.9) | 76 (79.2) | 0.19 (−0.04, 0.40) | 0.059 |
| Wine | 29 (30.2) | 26 (27.1) | 0.72 (0.55, 0.86) | <0.001 |
| Pulses | 12 (12.5) | 12 (12.5) | −0.05 (−0.17, 0.15) | 0.641 |
| Fish and seafood | 49 (51.0) | 39 (40.6) | 0.17 (−0.02, 0.35) | 0.089 |
| Sweets and pastries | 22 (22.9) | 11 (11.5) | 0.03 (−0.15, 0.24) | 0.715 |
| Nuts | 32 (33.3) | 21 (21.9) | 0.26 (0.05, 0.44) | 0.009 |
| Preference for white over red meat | 73 (76.0) | 5 (5.2) | 0.03 (0.31, 0.33) | 0.197 |
| Sofrito | 10 (10.4) | 6 (6.3) | 0.32 (0.01, 0.02) | 0.015 |
| Dependent Variable | Unstandardised Regression Coefficient a | 95% CIs | p-Value |
|---|---|---|---|
| Body mass index, kg/m2 | −0.044 | −0.492, 0.404 | 0.847 |
| Waist circumference, cm | −0.102 | −1.263, 1.060 | 0.862 |
| Systolic blood pressure, mmHg | 0.229 | −1.742, 1.713 | 0.799 |
| Diastolic blood pressure, mmHg | 0.232 | −0.781, 1.246 | 0.650 |
| Total cholesterol, mmol/L | −0.024 | −0.151, 0.103 | 0.708 |
| LDL-cholesterol b, mmol/L | −0.013 | −0.032, 0.006 | 0.181 |
| HDL-cholesterol, mmol/L | −0.012 | −0.062, 0.038 | 0.632 |
| Non-HDL-cholesterol, mmol/L | −0.012 | −0.129, 0.105 | 0.839 |
| Triglycerides b, mmol/L | 0.010 | −0.013, 0.034 | 0.380 |
| Total:HDL-cholesterol ratio b | 0.002 | −0.011, 0.016 | 0.731 |
| Triglyceride:HDL-cholesterol ratio b | 0.015 | −0.016, 0.047 | 0.340 |
| HbA1c b, % | 0.006 | −0.008, 0.020 | 0.400 |
| 1st Tertile (score = 0–4, n = 32) | 2nd Tertile (score = 5–6, n = 34) | 3rd Tertile (score = 7–14, n = 30) | p-Linear Trend b | |
|---|---|---|---|---|
| Foods | ||||
| Olive oil (g/4.18 MJ) | 0.6 (0.1, 1.1) | 2.2 (1.1, 3.5) | 4.3 (2.5, 6.7) | 0.001 |
| Vegetables (g/4.18 MJ) | 78.0 (57.6, 100.4) | 130.7 (103.6, 160.9) | 170.3 (132.3, 214.6) | 0.001 |
| Fruits (g/4.18 MJ) | 87.5 (57.2, 128.9) | 103.6 (74.5, 145.0) | 154.1 (110.0, 206.2) | 0.085 |
| Red and processed meat (g/4.18 MJ) | 70.1 (50.0, 90.1) | 39.8 (29.9, 50.5) | 32.7 (21.6, 44.3) | 0.001 |
| Butter and animal fats (g/4.18 MJ) | 11.0 (7.5, 14.7) | 10.3 (7.1, 13.8) | 7.0 (4.7, 9.6) | 0.101 |
| Sugar-sweetened beverages (g/4.18 MJ) | 77.8 (39.5, 119.4) | 18.3 (8.6, 30.7) | 9.1 (0.2, 23.1) | 0.001 |
| Wine (g/4.18 MJ) | 43.0 (16.9, 78.6) | 52.6 (28.7, 80.0) | 49.5 (24.7, 77.2) | 0.967 |
| Pulses (g/4.18 MJ) | 22.8 (14.8, 32.5) | 15.9 (8.6, 25.7) | 32.3 (9.5, 66.2) | 0.410 |
| Nuts (g/4.18 MJ) | 2.4 (0.8, 4.4) | 3.6 (1.6, 6.3) | 4.9 (2.4, 7.6) | 0.103 |
| Fish (g/4.18 MJ) | 34.0 (21.3, 49.1) | 28.8 (21.0, 37.0) | 49.8 (36.9, 64.0) | 0.094 |
| Nutrients | ||||
| Protein (% of energy intake) | 16.4 (15.2, 17.6) | 17.2 (15.6, 19.1) | 17.9 (16.6, 19.2) | 0.086 |
| Carbohydrates (% of energy intake) | 47.8 (44.9, 50.7) | 42.3 (39.6, 44.8) | 41.8 (38.9, 44.7) | 0.007 |
| Total fat (% of energy intake) | 33.7 (31.6, 35.8) | 38.1 (35.6, 40.8) | 37.9 (35.3, 40.9) | 0.021 |
| Saturated fat (% of energy intake) | 12.8 (11.7, 13.9) | 13.5 (12.1, 15.2) | 11.6 (10.3, 13.2) | 0.364 |
| Trans fat (% of energy intake) | 0.6 (0.5, 0.7) | 0.7 (0.5, 0.8) | 0.5 (0.4, 0.6) | 0.511 |
| Monounsaturated fat (% of energy intake) | 12.1 (11.2, 13.0) | 14.2 (13.0, 15.6) | 15.0 (13.8, 16.3) | 0.002 |
| Polyunsaturated fat (% of energy intake) | 4.6 (4.0, 5.3) | 4.5 (3.9, 5.2) | 5.7 (5.0, 6.5) | 0.022 |
| Dietary fibre (g/4.18 MJ) | 11.0 (9.7, 12.6) | 11.7 (10.5, 13.1) | 13.5 (12.2, 15.3) | 0.099 |
| Dietary cholesterol (mg/4.18 MJ) | 143.6 (123.4, 162.9) | 166.0 (138.6, 193.9) | 157.0 (128.1, 188.1) | 0.279 |
| Carotenes (ug/4.18 MJ) | 1396.6 (985.7, 1854.9) | 2488.3 (1747.6, 3299.4) | 2275.5 (1544.1, 3165.6) | 0.179 |
| Vitamin E (mg/4.18 MJ) | 4.8 (4.2, 5.4) | 5.1 (4.6, 5.6) | 6.3 (5.7, 6.8) | 0.001 |
| Folate (ug/4.18 MJ) | 141.4 (126.1, 159.1) | 154.6 (138.4, 171.0) | 164.7 (151.0, 179.9) | 0.107 |
| Vitamin C (mg/4.18 MJ) | 69.5 (49.9, 90.3) | 71.6 (56.7, 89.0) | 81.8 (67.1, 98.4) | 0.711 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, A.; Johnson, L.; Toumpakari, Z.; England, C.; Rai, M.; Toms, S.; Penfold, C.; Zazpe, I.; Martínez-González, M.A.; Feder, G. Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK. Nutrients 2018, 10, 138. https://doi.org/10.3390/nu10020138
Papadaki A, Johnson L, Toumpakari Z, England C, Rai M, Toms S, Penfold C, Zazpe I, Martínez-González MA, Feder G. Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK. Nutrients. 2018; 10(2):138. https://doi.org/10.3390/nu10020138
Chicago/Turabian StylePapadaki, Angeliki, Laura Johnson, Zoi Toumpakari, Clare England, Manmita Rai, Stu Toms, Chris Penfold, Itziar Zazpe, Miguel A. Martínez-González, and Gene Feder. 2018. "Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK" Nutrients 10, no. 2: 138. https://doi.org/10.3390/nu10020138






