The Influence of Pre-Exercise Glucose versus Fructose Ingestion on Subsequent Postprandial Lipemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preliminary Measurements
2.3. Test Drink, Oral Fat Tolerance Test, and Lunch and Dinner before Experiment Day
2.4. Protocol
2.5. Blood Sample Collection and Analysis
2.6. Statistical Analyses
3. Results
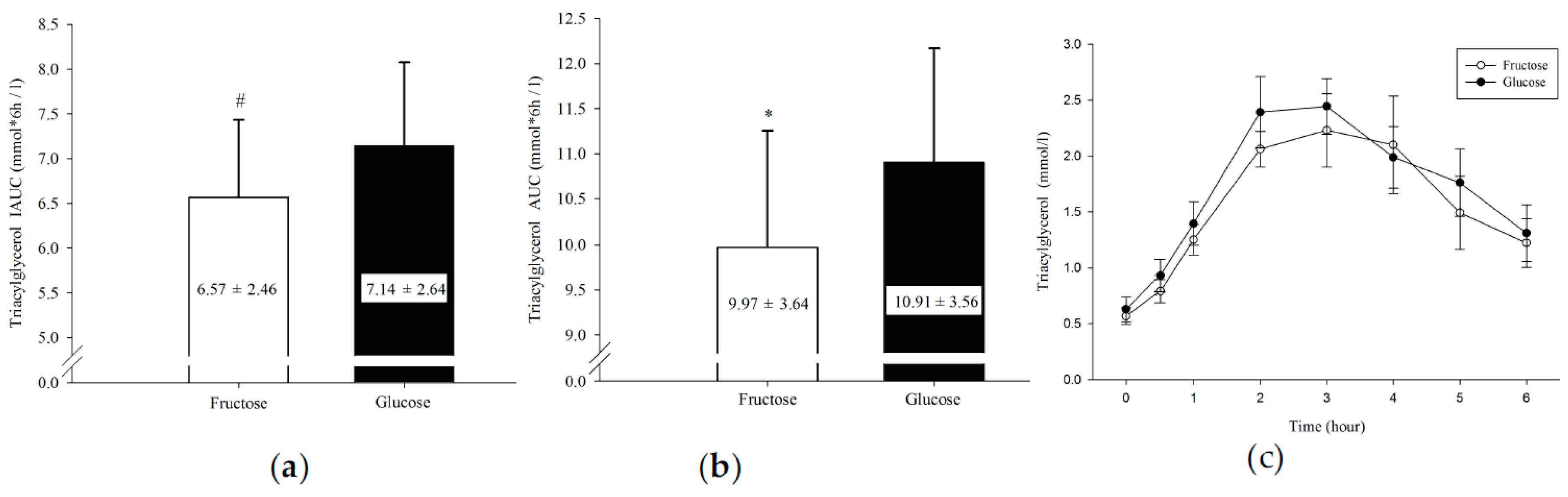
3.1. Plasma Triacylglycerol
3.2. Serum Insulin and Plasma Glucose
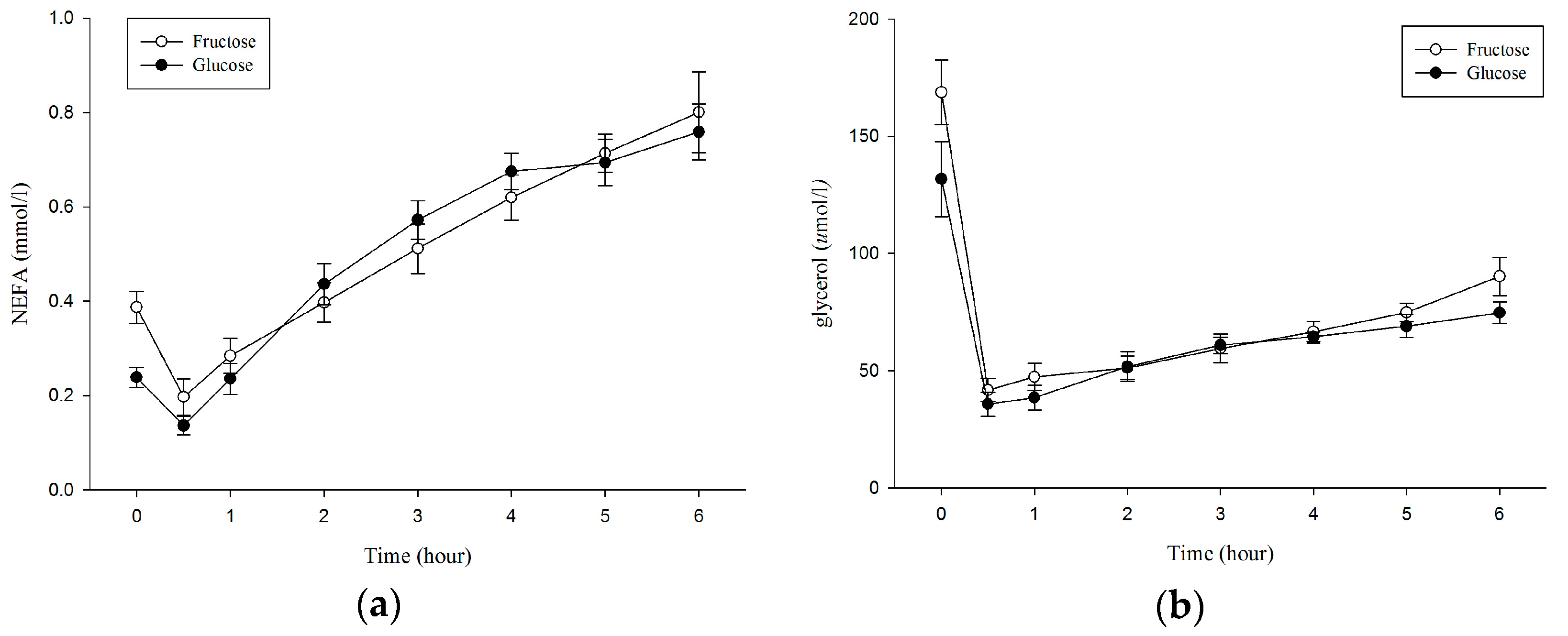
3.3. Plasma Non–Esterified Fatty Acids (NEFA), Glycerol
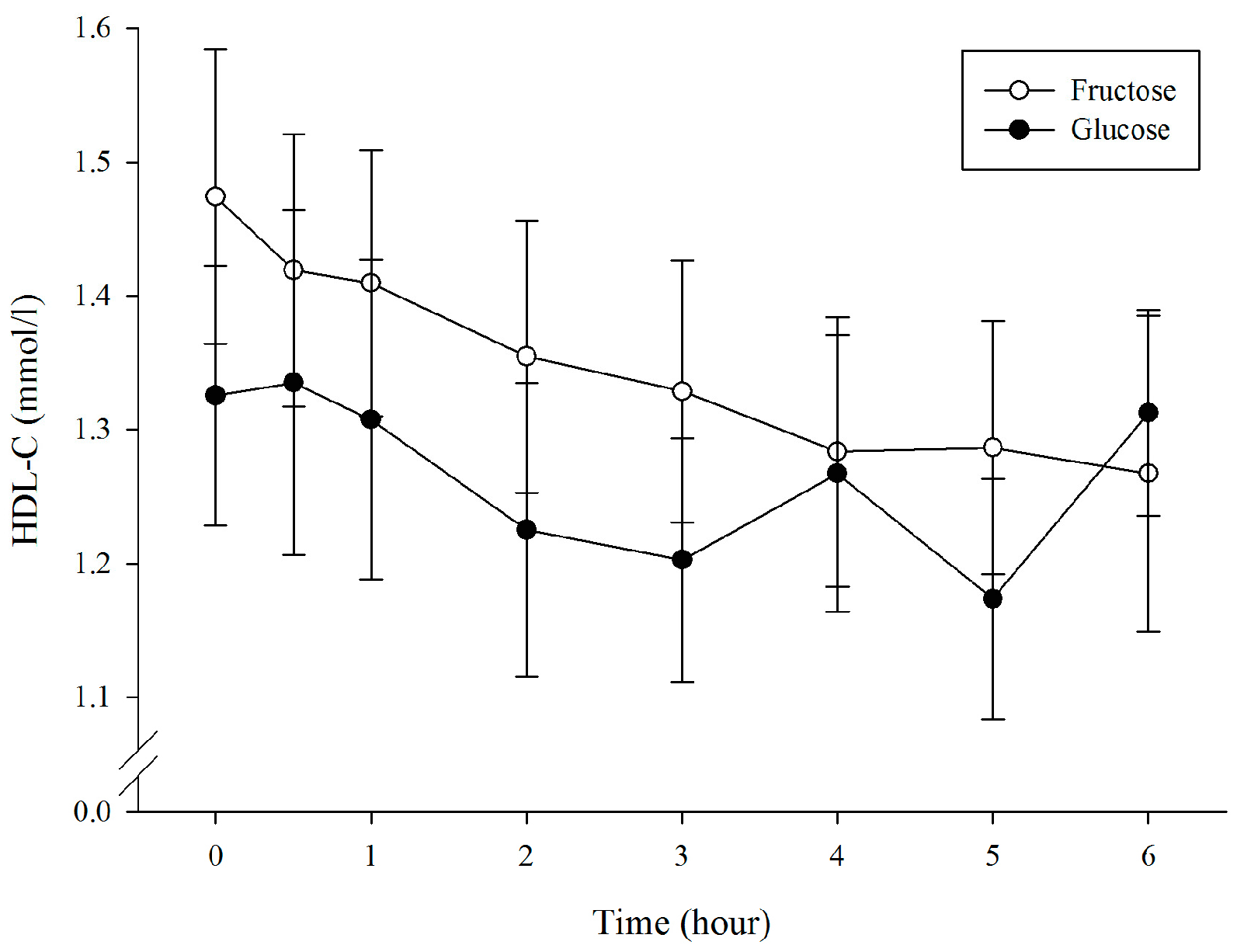
3.4. Plasma High-Density Lipoprotein Cholesterol
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lefebvre, P.J.; Scheen, A.J. The postprandial state and risk of cardiovascular disease. Diabet. Med. 1998, 15, S63–S68. [Google Scholar] [CrossRef]
- Chan, D.C.; Pang, J.; Romic, G.; Watts, G.F. Postprandial hypertriglyceridemia and cardiovascular disease: Current and future therapies. Curr. Atheroscler. Rep. 2013, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.R.; Miesenbock, G.; Hopferwieser, T.; Muhlberger, V.; Knapp, E.; Dunn, J.K.; Gotto, A.M., Jr.; Patsch, W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb. 1992, 12, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Sharrett, A.R.; Chambless, L.E.; Heiss, G.; Paton, C.C.; Patsch, W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Aldred, H.E.; Perry, I.C.; Hardman, A.E. The effect of a single bout of brisk walking on postprandial lipemia in normolipidemic young adults. Metabolism 1994, 43, 836–841. [Google Scholar] [CrossRef]
- Gill, J.M.; Al-Mamari, A.; Ferrell, W.R.; Cleland, S.J.; Sattar, N.; Packard, C.J.; Petrie, J.R.; Caslake, M.J. Effects of a moderate exercise session on postprandial lipoproteins, apolipoproteins and lipoprotein remnants in middle-aged men. Atherosclerosis 2006, 185, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tsetsonis, N.V.; Hardman, A.E.; Mastana, S.S. Acute effects of exercise on postprandial lipemia: A comparative study in trained and untrained middle-aged women. Am. J. Clin. Nutr. 1997, 65, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Hawley, J.A.; Jeukendrup, A. Pre-exercise carbohydrate and fat ingestion: Effects on metabolism and performance. J. Sports Sci. 2004, 22, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Sherman, W.M.; Dernbach, A.R. Carbohydrate feedings before, during, or in combination improve cycling endurance performance. J. Appl. Phys. 1991, 71, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J. Effect of dietary carbohydrate on triglyceride metabolism in humans. J. Nutr. 2001, 131, 2772S–2774S. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Park, S.; Choue, R. Comparison of time course changes in blood glucose, insulin and lipids between high carbohydrate and high fat meals in healthy young women. Nutr. Res. Pract. 2009, 3, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; McLaughlin, T.; Lamendola, C.; Kim, H.S.; Tanaka, A.; Wang, T.; Nakajima, K.; Reaven, G.M. High carbohydrate diets, triglyceride-rich lipoproteins, and coronary heart disease risk. Am. J. Cardiol. 2000, 85, 45–48. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Etienne, J.; McClure, W.C.; Pavey, B.S.; Holloway, A.K. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am. J. Physiol. 1998, 275, E1016–E1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Thomas, T.R.; Ball, S.D. Effect of exercise timing on postprandial lipemia and HDL cholesterol subfractions. J. Appl. Phys. 1998, 85, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Grandjean, P.W.; Moffatt, R.J. Effects of low and moderate exercise intensity on postprandial lipemia and postheparin plasma lipoprotein lipase activity in physically active men. J. Appl. Physiol. 2004, 96, 181–188. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Abbasi, F.; Lamendola, C.; Yeni-Komshian, H.; Reaven, G. Carbohydrate-induced hypertriglyceridemia: An insight into the link between plasma insulin and triglyceride concentrations. J. Clin. Endocrinol. Metab. 2000, 85, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Farquhar, J.W.; Reaven, G.M. Reappraisal of the role of insulin in hypertriglyceridemia. Am. J. Med. 1974, 57, 551–560. [Google Scholar] [CrossRef]
- Stevenson, E.J.; Williams, C.; Mash, L.E.; Phillips, B.; Nute, M.L. Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am. J. Clin. Nutr. 2006, 84, 354–360. [Google Scholar] [PubMed]
- Wee, S.L.; Williams, C.; Tsintzas, K.; Boobis, L. Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise. J. Appl. Physiol. 2005, 99, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Nicholas, C.; Williams, C.; Took, A.; Hardy, L. The influence of high-carbohydrate meals with different glycaemic indices on substrate utilisation during subsequent exercise. Br. J. Nutr. 2003, 90, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Chilibeck, P.D.; Yee, P.; Zello, G.A. The effect of consuming low-versus high-glycemic index meals after exercise on postprandial blood lipid response following a next-day high-fat meal. Nutr. Diabetes 2016, 6, e216. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.M.; Herd, S.L.; Vora, V.; Hardman, A.E. Effects of a brisk walk on lipoprotein lipase activity and plasma triglyceride concentrations in the fasted and postprandial states. Eur. J. Appl. Physiol. 2003, 89, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Koutsari, C.; Hardman, A.E. Exercise prevents the augmentation of postprandial lipaemia attributable to a low-fat high-carbohydrate diet. Br. J. Nutr. 2001, 86, 197–205. [Google Scholar] [PubMed]
- Chiu, C.H.; Burns, S.F.; Yang, T.J.; Chang, Y.H.; Chen, Y.L.; Chang, C.K.; Wu, C.L. Energy replacement using glucose does not increase postprandial lipemia after moderate intensity exercise. Lipids Health Dis. 2014, 13, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.L.; Williams, C. A low glycemic index meal before exercise improves endurance running capacity in men. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Wood, R.; Matthews, T.; Vanlangen, D.; Sawyer, J.; Headley, S. Substrate Utilization is Influenced by Acute Dietary Carbohydrate Intake in Active, Healthy Females. J. Sports Sci. Med. 2011, 10, 59–65. [Google Scholar] [PubMed]
- Campbell, M.D.; Walker, M.; Ajjan, R.A.; Birch, K.M.; Gonzalez, J.T.; West, D.J. An additional bolus of rapid-acting insulin to normalise postprandial cardiovascular risk factors following a high-carbohydrate high-fat meal in patients with type 1 diabetes: A randomised controlled trial. Diabetes Vasc. Dis. Res. 2017, 14, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Wright, D.C.; Patterson, B.W.; Mohammed, B.S.; Mittendorfer, B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E355–E362. [Google Scholar] [CrossRef] [PubMed]
- Tsekouras, Y.E.; Yanni, A.E.; Bougatsas, D.; Kavouras, S.A.; Sidossis, L.S. A single bout of brisk walking increases basal very low-density lipoprotein triacylglycerol clearance in young men. Metabolism 2007, 56, 1037–1043. [Google Scholar] [PubMed]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [PubMed]
- Bidwell, A.J.; Fairchild, T.J.; Redmond, J.; Wang, L.; Keslacy, S.; Kanaley, J.A. Physical activity offsets the negative effects of a high-fructose diet. Med. Sci. Sports Exerc. 2014, 46, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Egli, L.; Lecoultre, V.; Theytaz, F.; Campos, V.; Hodson, L.; Schneiter, P.; Mittendorfer, B.; Patterson, B.W.; Fielding, B.A.; Gerber, P.A.; et al. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes 2013, 62, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Malkova, D.; Evans, R.D.; Frayn, K.N.; Humphreys, S.M.; Jones, P.R.; Hardman, A.E. Prior exercise and postprandial substrate extraction across the human leg. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1020–E1028. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Lithell, H. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J. Clin. Investig. 1989, 83, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Richter, E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am. J. Physiol. 1998, 275, E332–E337. [Google Scholar] [CrossRef] [PubMed]
- Seip, R.L.; Angelopoulos, T.J.; Semenkovich, C.F. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am. J. Physiol. 1995, 268, E229–E236. [Google Scholar] [CrossRef] [PubMed]
- Pollare, T.; Vessby, B.; Lithell, H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler. Thromb. Vasc. Biol. 1991, 11, 1192–1203. [Google Scholar] [CrossRef]
- Jacobs, I.; Lithell, H.; Karlsson, J. Dietary effects on glycogen and lipoprotein lipase activity in skeletal muscle in man. Acta Physiol. Scand. 1982, 115, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lithell, H.; Jacobs, I.; Vessby, B.; Hellsing, K.; Karlsson, J. Decrease of lipoprotein lipase activity in skeletal muscle in man during a short-term carbohydrate-rich dietary regime. With special reference to HDL-cholesterol, apolipoprotein and insulin concentrations. Metabolism 1982, 31, 994–998. [Google Scholar] [CrossRef]
- Seip, R.L.; Mair, K.; Cole, T.G.; Semenkovich, C.F. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am. J. Physiol. 1997, 272, E255–E261. [Google Scholar] [CrossRef] [PubMed]
- Lithell, H.; Karlstrom, B.; Selinus, I.; Vessby, B.; Fellstrom, B. Is muscle lipoprotein lipase inactivated by ordinary amounts of dietary carbohydrates? Hum. Nutr. Clin. Nutr. 1985, 39, 289–295. [Google Scholar] [PubMed]
- Decombaz, J.; Sartori, D.; Arnaud, M.J.; Thelin, A.L.; Schurch, P.; Howald, H. Oxidation and metabolic effects of fructose or glucose ingested before exercise. Int. J. Sports Med. 1985, 6, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, V.A.; Karonen, S.L.; Nikkila, E.A. Carbohydrate ingestion before exercise: Comparison of glucose, fructose, and sweet placebo. J. Appl. Physiol. 1981, 51, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, D.; Peronnet, F.; Allah, C.; Hillaire-Marcel, C.; Ledoux, M.; Brisson, G. Metabolic response to [13C]glucose and [13C]fructose ingestion during exercise. J. Appl. Physiol. 1986, 61, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Blazek, A.; Rutsky, J.; Osei, K.; Maiseyeu, A.; Rajagopalan, S. Exercise-mediated changes in high-density lipoprotein: Impact on form and function. Am. Heart J. 2013, 166, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.W.; Crouse, S.F.; Rohack, J.J. Influence of cholesterol status on blood lipid and lipoprotein enzyme responses to aerobic exercise. J. Appl. Physiol. 2000, 89, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Laws, A.; Reaven, G.M. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J. Intern. Med. 1992, 231, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Sharman, M.J.; Gomez, A.L.; DiPasquale, C.; Roti, M.; Pumerantz, A.; Kraemer, W.J. Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. J. Am. Coll. Nutr. 2004, 23, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Sharman, M.J.; Gomez, A.L.; Scheett, T.P.; Kraemer, W.J. An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial lipemic responses compared with a low fat diet in normal weight, normolipidemic women. J. Nutr. 2003, 133, 2756–2761. [Google Scholar] [PubMed]

| Fructose | Glucose | p | ES | |
|---|---|---|---|---|
| Insulin (μU/mL × 6 h) | 74.06 ± 20.95 | 71.73 ± 17.88 | 0.717 | 0.12 |
| TG (mmol/L × 6 h) | 9.97 ± 3.64 | 10.91 ± 3.56 | 0.033 * | 0.26 |
| TG IAUC(mmol/L × 6 h) | 6.57 ± 2.46 | 7.14 ± 2.64 | 0.004 * | 0.22 |
| Glucose (mmol/L × 6 h) | 27.46 ± 3.30 | 27.56 ± 1.59 | 0.951 | 0.04 |
| NEFA (mmol/L × 6 h) | 3.05 ± 0.45 | 3.06 ± 0.54 | 0.962 | 0.02 |
| Glycerol (μmol/L × 6 h) | 395.84 ± 69.55 | 363.19 ± 64.67 | 0.192 | 0.49 |
| HDL-C (mmol/L × 6 h) | 8.02 ± 1.68 | 7.49 ± 1.52 | 0.003 * | 0.33 |
| Fructose | Glucose | p | ES | |
|---|---|---|---|---|
| Insulin (μU/mL) | 4.78 ± 3.86 | 8.50 ± 6.31 | 0.177 | 0.71 |
| TG (mmol/L) | 0.57 ± 0.21 | 0.63 ± 0.31 | 0.580 | 0.23 |
| Glucose (mmol/L) | 4.52 ± 0.69 | 5.29 ± 1.22 | 0.101 | 0.78 |
| NEFA (mmol/L) | 0.39 ± 0.10 | 0.24 ± 0.06 | 0.011 * | 1.82 |
| Glycerol (μmol/L) | 168.8 ± 38.86 | 131.7 ± 45.30 | 0.015 * | 0.88 |
| HDL-C (mmol/L) | 1.47 ± 0.31 | 1.33 ± 0.27 | 0.184 | 0.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-J.; Chiu, C.-H.; Tseng, M.-H.; Chang, C.-K.; Wu, C.-L. The Influence of Pre-Exercise Glucose versus Fructose Ingestion on Subsequent Postprandial Lipemia. Nutrients 2018, 10, 149. https://doi.org/10.3390/nu10020149
Yang T-J, Chiu C-H, Tseng M-H, Chang C-K, Wu C-L. The Influence of Pre-Exercise Glucose versus Fructose Ingestion on Subsequent Postprandial Lipemia. Nutrients. 2018; 10(2):149. https://doi.org/10.3390/nu10020149
Chicago/Turabian StyleYang, Tsung-Jen, Chih-Hui Chiu, Mei-Hui Tseng, Cheng-Kang Chang, and Ching-Lin Wu. 2018. "The Influence of Pre-Exercise Glucose versus Fructose Ingestion on Subsequent Postprandial Lipemia" Nutrients 10, no. 2: 149. https://doi.org/10.3390/nu10020149





