1. Introduction
Iron and vitamin D are two essential nutrients which constitute an important worldwide health issue due to their significant roles in biochemistry and simultaneously, the very high risk of deficiency in both of them [
1,
2].
Vitamin D plays a dual role in the human body as a prohormone nutrient and fat soluble vitamin. Due to its pleiotropic nature, beyond its influence on bone health, vitamin D demonstrates significant involvement in various gene expression processes and plays key roles in calcium and phosphate metabolism, which are involved in a multitude of physiological and pathophysiological mechanisms [
3]. Deficiency in vitamin D is linked to numerous illnesses and pathological conditions, including musculoskeletal health, immunity, cardiovascular disease, cancer and mental health [
4], as well as deterioration of athletic performance [
5,
6,
7]. The high prevalence of low serum vitamin D concentration is a global problem in all age groups, even in regions of high sun exposure [
8]. Athletes appear to have a similar risk of vitamin D deficiency as nonathletic subjects from the same population. Seasonal variance in vitamin D status is observed in athletes as well as in the general population [
9,
10]. However, it should be noted that exercise-induced stress may also promote deterioration of vitamin D levels, especially in athletes training and competing indoors [
5,
6,
7,
11,
12].
Iron is another essential nutrient which is involved in many physiological processes, particularly in the production of red blood cells and myoglobin, oxygen transport and the production of ATP, DNA synthesis, and electron transport in mitochondria [
1,
13,
14]. Although the human system has created mechanisms for preventing iron deficiency, the lack of this mineral is one of the basic factors associated with anemia [
15]. Approximately 50% of all anemia cases in developed countries are caused by iron deficiency [
2]. The groups particularly exposed to deficiencies of this mineral are women of reproductive age, children and adolescents [
2,
16,
17]. Results from many studies indicate that athletes are also at high risk for iron deficiency [
18,
19,
20], and this applies especially to physically active women [
19,
21,
22].
Numerous cross-sectional studies have indicated an association between low 25(OH)D concentration and poor iron status [
23,
24,
25,
26]. Furthermore, Azizi-Soleiman [
1], in a systematic review, pointed out that such relationships may be mutual. It is known that a deficit of vitamin D may cause deterioration of iron status [
27,
28] and increase the risk of anemia [
26,
29,
30]. The precise mechanisms for this dependence are still not understood [
26], but it is hypothesized that vitamin D may affect iron regulation and erythropoiesis by its influence on hepcidin via cytokines [
31,
32] or independently of changes in pro-inflammatory markers [
33,
34]. There are also findings indicating that vitamin D may directly influence erythroid precursors in bone marrow [
23,
35].
Iron deficiency, in turn, was identified as one of the factors for vitamin D deficiency. The positive correlation between these two nutrients is confirmed by an increase of vitamin D concentration after intramuscular iron treatment in infants [
36] as well as a positive correlation between hematological and non-heme indices of iron status with 25(OH)D concentration [
24,
36,
37], although the exact mechanisms of this dependence are also not known. There is evidence that a deficit in iron may disturb the synthesis of vitamin D
3 and lead to its mild deficiency, because conversion of cholecalciferol to the biologically active form, calcitriol (1,25-dihydroxyvitamin D
3) requires two steps of hydroxylation—the first in the liver and the second in the kidney—which depend on enzymes containing heme, i.e., cytochromes P450 (CYP2R1 and CYP27B1 respectively) [
38,
39].
Both nutrients have frequently drawn the attention of researchers, and there is a wealth of data on athletes concerning either the assessment of vitamin D [
10,
12,
40] or iron status in athletes [
19,
40,
41]; however, there are still few studies examining the interdependence between them. So far, only Constantini et al. [
27] have analyzed the relationship between both of these nutritional components. They observed the influence of vitamin D levels on iron and serum ferritin concentrations. Moreover, there is a lack of research investigating the impact of iron on vitamin D status in physically active people. To the best of our knowledge, there is no research in which the mutual relationship between these two nutrients has been examined.
Since female athletes are a group at particularly high risk of both vitamin D and iron deficiency, it seemed sensible to examine: (1) whether deficiencies of vitamin D are associated with reduced iron status and (2) whether progressive iron deficiency is accompanied by inferior vitamin D status.
3. Results
Mean values (±SD) and ranges of all studied indices in the whole group of female athletes are presented in
Table 2.
The frequency of female athletes with 25(OH)D concentration below 75 nmol/L was 54.3%, wherein most subjects showed insufficient concentrations (within 25–75 nmol/L). A deficit of this vitamin (<25 nmol/L) was only observed in 1.8% of females.
Total iron deficiency was identified in 23.3% of female athletes. Low iron stores were observed in 7.3%, latent ID in 15.1% and iron deficiency anemia in 0.9% of subjects.
Logistic regression analysis (
Table 3), expressed as the odds ratio (OR), with a 95% confidence interval (95%CI), indicated that in female athletes, vitamin D deficiency was significantly (
p = 0.01) affected by two factors: the length of day (OR = 2.29; 95% CI 1.28–4.07;
p = 0.005) and iron deficiency (OR = 2.96; 95% CI 1.45–6.02;
p = 0.003). Iron deficiency, in turn, was correlated with vitamin D deficiency (OR = 2.73; 95% CI 1.32–5.62;
p = 0.007) and age (OR = 0.82; 95% CI 0.73–0.91;
p = 0.000).
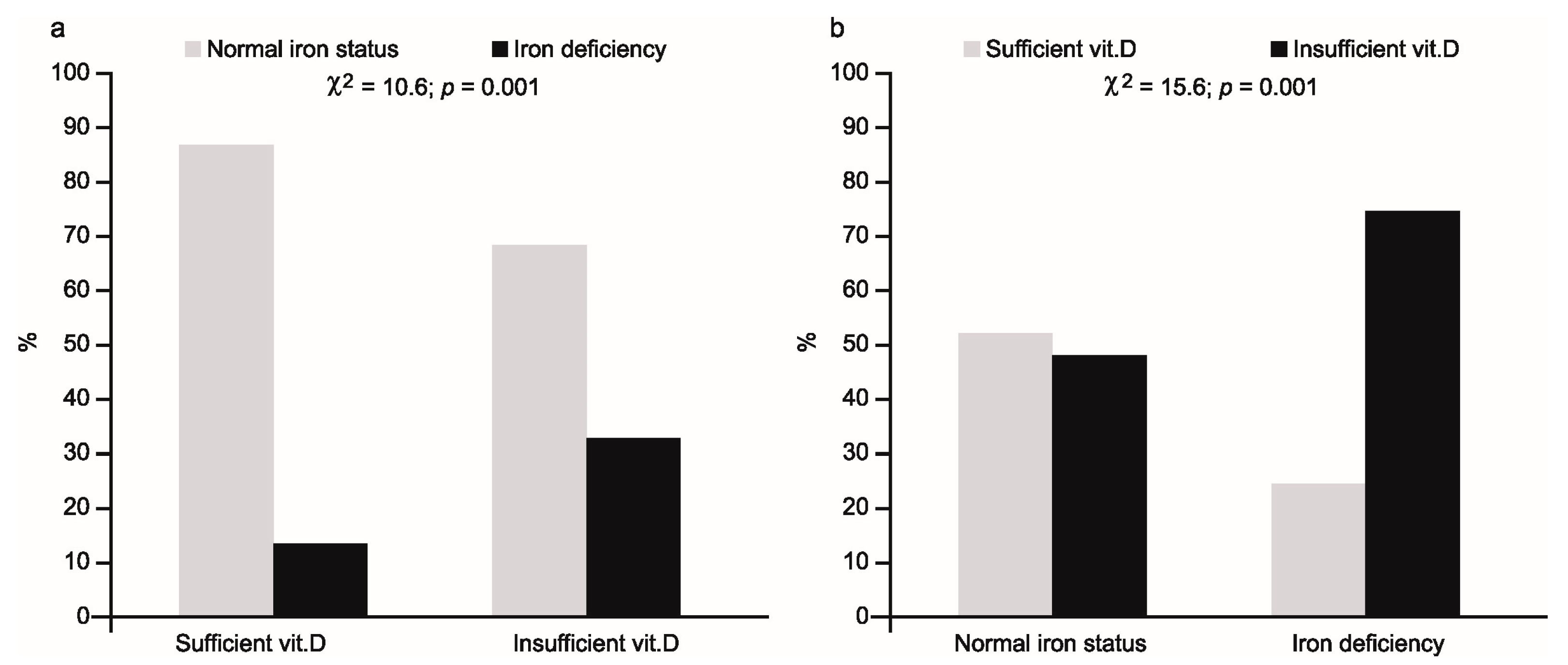
The percentage of athletes with iron deficiency and normal iron status in relation to vitamin D status ((a)—left part of figure) and vice versa ((b)—right part of figure) is shown in
Figure 1.
In the first case, iron deficiency was present in 32% of 25(OH)D deficient subjects, compared with 11% in the vitamin sufficient group (χ2 = 10.6; p = 0.001). Conversely, low 25(OH)D concentration was observed in 75% of iron deficient females, compared with 48% of subjects with normal iron status (χ2 = 15.6; p = 0.001).
The odds ratios for vitamin D deficiency were significantly higher for those with iron deficiency, and the values of ORs increased along with a decreasing level of ferritin as a criterion for iron deficiency, from 1.75 (95% CI 1.02–2.99;
p = 0.040) at a ferritin level of 30 µg/L to 3.56 (95% CI 1.6–3.12;
p = 0.002) at a ferritin level of 12 µg/L. The highest value of OR equal to 4.6 (95% CI 1.81–11.65;
p = 0.001) was observed in the group with more advanced iron deficiency, i.e., in subjects with latent ID and IDA (
Table 4).
The ORs for iron deficiency were significantly higher for both the subjects with concentrations of 25(OH)D < 75 nmol/L and for those with concentrations of <50 nmol/L (
Table 4). The ORs for those groups were 3.14 (95% CI 1.56–6.31;
p = 0.001) and 3.18 (95% CI 1.09–9.26;
p = 0.030), respectively, and did not differ between each other.
In athletes with iron deficiency, significantly lower mean serum 25(OH)D concentrations (
p = 0.000) were observed (
Table 5), while the group with insufficient vitamin D concentrations had significantly different values for all four indices of iron status: ferritin, iron, sTfR and TIBC (
Table 6).
The mean values of ferritin (
p = 0.043) and iron (
p = 0.004) were significantly lower, while the mean values of TIBC (
p = 0.016) and sTfR (
p = 0.001) were significantly higher, compared to the group with normal 25(OH)D concentration (
Table 6). Additionally, in the group with reduced vitamin D concentration, lower mean values of some hematological indices in reticulocytes (CHr,
p = 0.049 and MCVr,
p = 0.020) were observed, although the mean value of RBC in this group was higher (
p = 0.029).
4. Discussion
The high frequency of both iron and vitamin D deficiencies observed in the present study are in accordance with many earlier studies concerning physically active subjects [
10,
12,
19,
21,
22,
27,
43]. This confirms that the problem regarding deficiencies in both nutrients in athletes is still present.
With gradual recognition of the role of vitamin D deficiency in many diseases [
4], the relationship between vitamin D and iron status has also begun to be explored [
1]. It has been demonstrated that a deficit of vitamin D increases the risk of many hematological disorders and iron metabolism disturbances [
26,
44], which was visible, especially in adults with different illnesses [
26]. One reason for this is the pro-inflammatory effects of a vitamin D deficit, which eventually leads to an increase in hepcidin production, via stimulation of pro-inflammatory cytokines [
31] and activation of the JAK-STAT3 pathway [
44]. Sun et al. [
34] pointed out that vitamin D can also downregulate hepcidin transcription, although the mechanism by which this occurs is unknown. High hepcidin levels, in turn, may favor sequestration of iron in macrophages and hepatocytes, which promotes the development of inflammatory anemia [
32]. This anti-inflammatory effect of vitamin D is confirmed by studies pointing to the reduction in hepcidin levels and increase in 25(OH)D concentration in vitamin D deficient subjects after supplementation with this vitamin [
33].
The lack of association between vitamin D status and the two main hematology parameters used to diagnose anemia—Hb and HCT—and the higher mean values of RBC, in the group with reduced vitamin D concentrations in our study, may have resulted from the fact that only healthy athletes (without any symptoms of acute phase reaction) were studied. This confirms the hypothesis that vitamin D deficiency might be particularly associated with inflammatory anemia [
45], although it is worth emphasizing that a positive association between vitamin D and morphological parameters was also observed in healthy adults [
23].
The logistic regression analysis indicated, however, that in female athletes, in addition to age (lower age favors iron deficiency), vitamin D deficiency significantly increases the risk of ID (
Table 3). The reverse relationship between age and iron status in females is known [
46], but the impact of vitamin D on iron status is less proven, especially in terms of its effect on non-hematological parameters. Despite the association between vitamin D and blood morphology indices having been investigated relatively often, the results are contradictory, mainly due to the study of different subtypes of anemia [
47] and presence of inflammation [
23]. Furthermore, in some studies, only ferritin was measured [
27,
33] and non-heme indices were not tested at all [
48]. In our study the frequency of iron deficiency was significantly higher in the group with reduced 25(OH)D concentrations (
Figure 1). Simultaneously, in this group, we observed significant changes in all four indices of iron status. Positive relationships of ferritin and iron concentrations and negative relationships of TIBC values and sTfR concentrations (
Table 6) clearly indicated a worsening of both storage and transport iron pools in the body. In addition, in the group with reduced vitamin D levels, significantly lower values of two (independent of plasma volume) hematological parameters, including reticulocytes, were observed. A lower mean corpuscular volume (MCVr) and mean corpuscular hemoglobin concentration (CHr) in reticulocytes indicates that the deficit of iron in this group had started to affect the functional iron pool as well. This is logical because in the group with vitamin D deficiency, a relatively high percentage (about 76%) of subjects with iron deficiency manifested a more advanced deficit in iron (i.e., stage II iron deficiency) with two athletes having iron deficiency anemia. The observed, simultaneously opposite relationship between vitamin D status and RBC may be due to post-exercise changes in plasma volume [
49].
There are several possible mechanisms which could explain the impairment of iron status coexisting with vitamin D deficiency. One of them assumes that insufficient quantities of vitamin D may impair iron availability and its absorption, via an increase in hepcidin concentration, due to the increase in some cytokines—e.g., IL-6 or IL-1B [
31,
32]—which may also take place after physical effort [
50]. However, the results of the recent study by Smith et al. [
33] indicated that in healthy adults, vitamin D may act on hepcidin directly—that is, without cytokines. Moreover, hepcidin may not only hamper the availability of iron from monocytes, hepatocytes and enterocytes, through the iron–hepcidin–ferroportin axis [
31], but additionally, may impair absorption of iron due to a decrease in duodenal divalent metal transporter 1 (DMT1) levels [
51]. Even though we excluded all the subjects with any symptoms of an acute phase response, we unfortunately did not measure hepcidin and proinflammatory cytokine concentrations, which, as a consequence, did not allow for a more accurate analysis of the obtained results. In this situation, we can only presume that hepcidin may be involved in the deterioration of iron status, although a direct effect of vitamin D on red blood cell production is also possible. It has been reported that metabolites of vitamin D (especially its active form) are crucial for normal red blood cell production, via the stimulation of erythroid progenitor cells in a synergistic fashion with erythropoietin [
23,
35]. In bone marrow, the levels of 25(OH)D and (1,25(OH)
2D) are 25- and 500-fold higher, respectively, in comparison to serum [
52]. Low 25(OH)D levels in marrow tissue may lead to insufficient substrate availability for 1α-hydroxylase-induced synthesis of the active form of vitamin D, which is needed for hematopoiesis [
35].
It is worth noting that the risk of iron deficiency did not increase with decreasing 25(OH)D concentration (
Table 4). Similar OR values for both 75 and 50 nmol/L concentrations (3.14, 95% CI 1.56–6.31,
p = 0.001 and 3.18, 95% CI 1.09–9.26,
p = 0.030, respectively) indicated that a vitamin D concentration below 75 nmol/L may be impacted by worsening of the iron status. Our results are consistent with the results of others. Similar differences in OR values for 50 and 75 nmol/L were observed by Atkinson et al. [
48] in white children in the USA. Sim et al. [
23] also indicated 75 nmol/L as a limit value, although there are data supporting a lower threshold value (i.e., 50 nmol/L) below which the risk of anemia becomes clearly higher [
29].
Whereas the impact of vitamin D deficiency on blood morphology and non-hematological iron status indices has been studied fairly often, the inverse association between these two nutrients has been tested less often. Some studies concerning bone tissue indicate that iron deficiency is a risk factor for both impaired vitamin D and bone metabolism in humans [
37,
38]. The present results are in line with this relationship, because, in our study, iron deficiency was also related to the worsening of vitamin D status. This was confirmed by the multivariable-adjusted logistic regression model, which indicated iron status (OR = 2.96, 95% CI 1.45–6.02,
p < 0.003) and length of day (OR = 2.29, 95% CI 1.28–4.07,
p < 0.005) as the two factors significantly affecting vitamin D status. Furthermore, among iron deficient subjects, the percentage of female athletes with a low 25(OH)D concentration was relatively high (75%)—significantly higher than in the group without iron deficiency (
Figure 1)—and the mean concentration of 25(OH)D was significantly (
p = 0.000) lower in the group showing iron deficiency (
Table 5).
Fluctuations in vitamin D due to latitude, weather pattern and length of solar exposure during summer are well known [
10,
28], but the role of iron in vitamin D metabolism is less clear. Iron, as a component of the cytochrome P450 monooxygenase superfamily, participates in synthesis of the active form of vitamin D
3, not only in the last stage of its bioactivation from 25(OH)D to 1,25-dihydroxyvitamin D
3 (25-hydroxyvitamin D 1-α-hydroxylase—CYP27B1), but also in the earlier stage in which cholecalciferol is converted to 25(OH)D (25-hydroxylase—CYP2R1) [
38,
53]. Therefore, as a consequence of iron deficiency, activity of these iron-containing enzymes may be lowered, and hence, a deficit in vitamin D
3 may occur. This important role of iron in the synthesis of vitamin D was clearly confirmed by Katsumata et al. [
39], who reported that dietary iron deficiency in rats caused diminished 1α-hydroxylase activity, leading to a decrease in serum 1,25-dihydroxyvitamin D
3 concentration. The mentioned findings may explain why, in an earlier study, Heldenberg et al. [
36] showed that a single intramuscular injection of iron in infants with iron deficiency anemia resulted in an increase in 25(OH)D concentration. These facts may also explain why, in the present study, the concentration of the metabolite, 25(OH)D, was significantly lower in subjects with iron deficiency. Some observational studies indicate that iron deficiency may be a significant predictor of vitamin D level [
24,
27,
36]; however, there are some trial studies which showed that iron supplementation had no effect on 25(OH)D level [
37,
54]. Azizi-Soleiman [
1] and Katsumata et al. [
39] emphasize that the reason for this might be the degree of iron deficiency. So far, it is not known how severe iron deficiency must be to impair vitamin D synthesis, so we attempted to determine the value of ferritin concentration at which the risk of vitamin D deficiency starts to significantly increase. The ORs calculated for different values of ferritin (from 30 to 12 µg/L) indicate that the risk of vitamin D deficiency started to be significant at ferritin concentrations below 30 µg/L. The gradual increase in OR, from 1.75 (95% CI 1.02–2.99) at 30 µg/L ferritin to 4.60 (95% CI 1.81–11.65), in athletes with stage II ID and IDA suggests that disorders in vitamin D synthesis can arise along with worsening iron status.
The present study has some strengths but also some limitations. The strengths are as follows: the availability of data from healthy young athletes only, a relatively high number of subjects with iron and vitamin D deficiencies and the opportunity to perform a multivariable-adjusted logistic regression analysis, taking into account some confounders (age, season of blood collection, as well as in- and outdoor sports disciplines). Regarding limitations, first, the cross-sectional study design did not allow us to draw definite conclusions concerning causal relationships between studied nutrients. Another limitation is the lack of international, unequivocal threshold values for diagnosing both vitamin D and iron deficiency. Furthermore, as mentioned above, the lack of data on hepcidin and interleukins did not allow for a more accurate explanation of the presumed anti-inflammatory effect of vitamin D on iron status in physically active people. Lastly, we also lacked data on time spent outdoors (sun exposure), nutrient intake and vitamin D and iron supplementation, because the study was performed during periodic medical examinations, and it was impossible to perform interviews regarding supplementation.