2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures
Abstract
:1. Introduction
2. Experimental Section
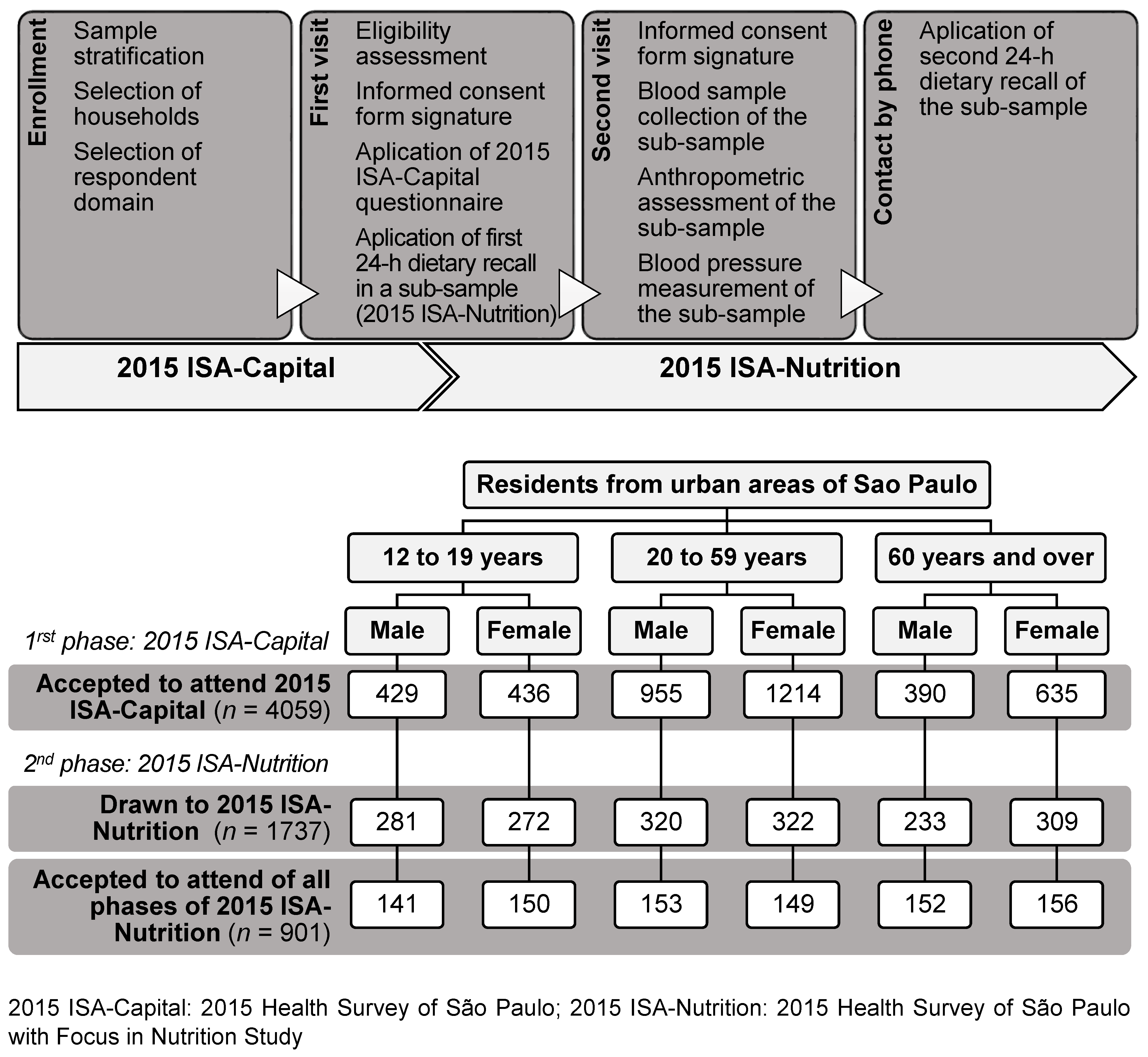
2.1. Study Population and Sampling Design
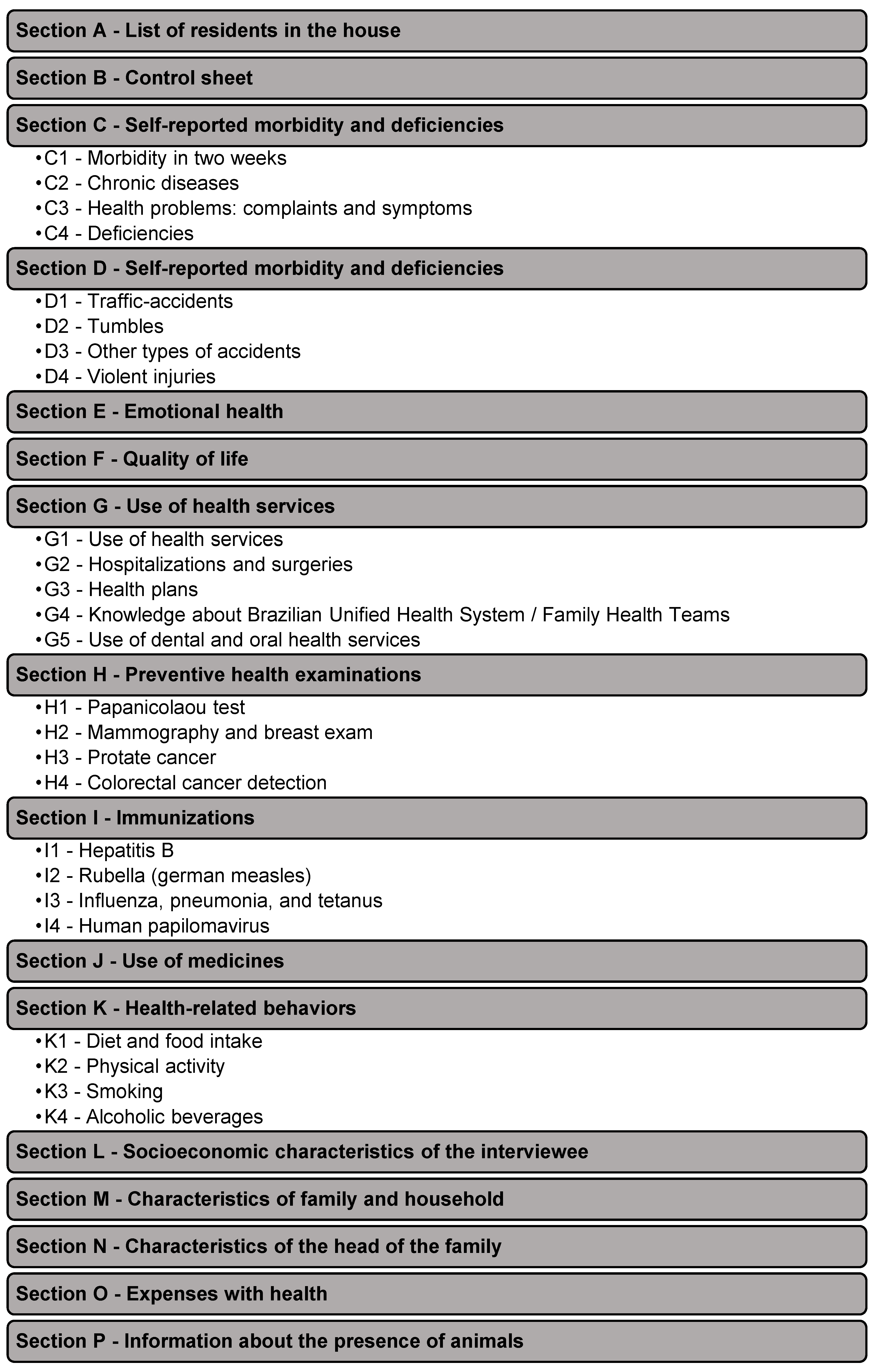
2.2. Study Design
2.3. Emotional Health
2.4. Physical Activity Practice
2.5. Alcohol Consumption Habits
2.6. Dietary Assessment
2.7. Anthropometric Measurements
2.8. Blood Pressure Measurement
2.9. Data management, Quality Assurance Strategies, and Dissemination Plans
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlson, F.J.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Hearts: Technical Package for Cardiovascular Disease Management in Primary Health Care; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Prefeitura de São Paulo. Inquérito de Saúde do Município de São Paulo (ISA-Capital). 2015. Available online: http://www.prefeitura.sp.gov.br/cidade/secretarias/saude/epidemiologia_e_informacao/isacapitalsp/index.php?p=216392 (accessed on 24 July 2017).
- Moschonis, G.; Tsoutsoulopoulou, K.; Efstathopoulou, E.; Tsirigoti, L.; Lambrinou, C.P.; Georgiou, A.; Filippou, C.; Lidoriki, I.; Reppas, K.; Androutsos, O.; et al. Conceptual framework of a simplified multi-dimensional model presenting the environmental and personal determinants of cardiometabolic risk behaviors in childhood. Expert Rev. Cardiovasc. Ther. 2015, 13, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Sing, C.F.; Stengård, J.H.; Kardia, S.L. Genes, environment, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Diem, G.; Brownson, R.C.; Grabauskas, V.; Shatchkute, A.; Stachenko, S. Prevention and control of noncommunicable diseases through evidence-based public health: Implementing the NCD 2020 action plan. Glob. Health Promot. 2016, 23, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.C.G.P.; Escuder, M.M.L.; Barros, M.B.A.; Goldbaum, M.; Fisberg, R.; Cesar, C.L.G. Plano de amostragem em inquéritos de saúde-município de São Paulo. Rev. Saude Publ. 2015, in press. [Google Scholar]
- Rodriguez, M.P.; Narizano, A.; Demczylo, V.; Cid, A. A simple method for the determination of zinc human-plasma levels by flame atomic absorption. At. Spectrosc. 1989, 10, 68–70. [Google Scholar]
- Mari, J.J.; Williams, P. A validity study of a psychiatric screening questionnaire (SRQ-20) in primary care in the city of Sao Paulo. Br. J. Psychiatry 1986, 148, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.F.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Matsudo, S.; Araújo, T.; Mtsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. Questionário internacional de atividade física (IPAQ): Estudo de validade e reprodutibilidade no Brasil. Atividade Física Saúde 2001, 6, 5–18. [Google Scholar]
- World Health Organization (WHO). Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Babor, T.; Higgins-Biddle, J.C.; Saunders, J.B.; Monteiro, M.G. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Health Care; Department of Mental Health and Substance Dependence, World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Lima, C.T.; Freire, A.C.C.; Silva, A.P.B.; Teixeira, R.M.; Farrell, M.; Prince, M. Concurrent and construct validity of the audit in an urban Brazilian sample. Alcohol Alcohol. 2005, 40, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s Dietary Intake Data System. J. Food Compost. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- NCC. Food and Nutrient Database Version 2014; Regents of the University of Minnesota—NDSR Manual: Minneapolis, MN, USA, 2014. [Google Scholar]
- Pinheiro, A.B.V.; Lacerda, E.M.A.; Benzecry, E.H.; Gomes, M.C.S.; Costa, V.M. Tabela Para Avaliação de Consumo Alimentar em Medidas Caseiras, 4th ed.; Atheneu: São Paulo, Brazil, 2000. [Google Scholar]
- Fisberg, R.M.; Villar, B.S. Manual de Receitas e Medidas Caseiras Para Cálculo de Inquéritos Alimentares: Manual Elaborado Para Auxiliar o Processamento de Inquéritos Alimentares; Signus: São Paulo, Brazil, 2002. [Google Scholar]
- Núcleo de Estudos e Pesquisas em Alimentação (NEPA), Universidade Estadual de Campinas. Tabela Brasileira de Composição de Alimentos, 4th ed.; NEPA-UNICAMP: Campinas, Brazil, 2011. [Google Scholar]
- Haubrock, J.; Nӧthlings, U.; Volatier, J.; Dekkers, A.; Ocke, M.; Harttig, U.; Illner, A.; Knϋppel, S.; Andersen, L.F.; Boeing, H. Estimating usual food intake distributions by using the Multiple Source Method in the EPIC-Potsdam Calibration Study. J. Nutr. 2011, 141, 914–920. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation (WHO Technical Report Series 894); World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organization (WHO). Growth Reference Data for 5–19 Years; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization (WHO). Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Lebrão, M.L.; Duarte, Y.A.O. SABE—Saúde, Bem-Estar e Envelhecimento—Projeto Sabe No Município de São Paulo: Uma Abordagem Inicial; Organização Pan-Americana da Saúde: Brasília, Brazil, 2003. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Section Name: Cidades. Available online: http://cidades.ibge.gov.br/xtras/perfil.php?codmun=355030 (accessed on 16 October 2017).
- Ikeda, N.; Takimoto, H.; Imai, S.; Miyachi, M.; Nishi, N. Data Resource Profile: The Japan National Health and Nutrition Survey (NHNS). Int. J. Epidemiol. 2015, 44, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.; Lennox, A.; Prentice, A.; Bates, C.; Page, P.; Nicholson, S.; Swan, G. National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009–2011/2012). Public Health Eng. 2014, 2014, 1–15. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de Orçamentos Familiares 2008–2009—Análise do Consumo Alimentar Pessoal; IBGE: Rio de Janeiro, Brazil, 2011.
- Ministério da Saúde; Secretaria de Vigilância em Saúde; Departamento de Doenças e Agravos não transmissíveis e Promoção da Saúde. Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico, Vigitel 2013; Ministério da Saúde: Brasília, Brazil, 2014.
- Szwarcwald, C.L.; Malta, D.C.; Pereira, C.A.; Vieira, M.L.F.P.; Conde, W.L.; Souza Junior, P.R.B.D.; Damacena, G.N.; Azevedo, L.O.; Azevedo E Silva, G.; Theme Filha, M.M.; et al. National Health Survey in Brazil: Design and methodology of application. Cienc. Saude Colet. 2014, 19, 333–342. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa Nacional por Amostra de Domicílios. Um Panorama da Saúde no Brasil. Acesso e Utilização de Serviços, Condições de Saúde e Fatores de Risco e Proteção à Saúde 2008; IBGE: Rio de Janeiro, Brazil, 2010.
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Aliete, D.; Pereira, J.L.; Fontanelli, M.; Carioca, A.; Marchioni, D.; Fisberg, R. Dietary underreporting: Comparison between face-to-face and telephone 24-hour recalls in Health Survey of São Paulo. In Proceedings of the 12th Europeans Nutrition Conference, Berlim, Germany, 20–23 October 2015. [Google Scholar]
- Castilho, E.A.; Goldbaum, M. Chronic noncommunicable diseases and population surveys. Rev. Saude Publ. 2017, 51. [Google Scholar] [CrossRef] [PubMed]
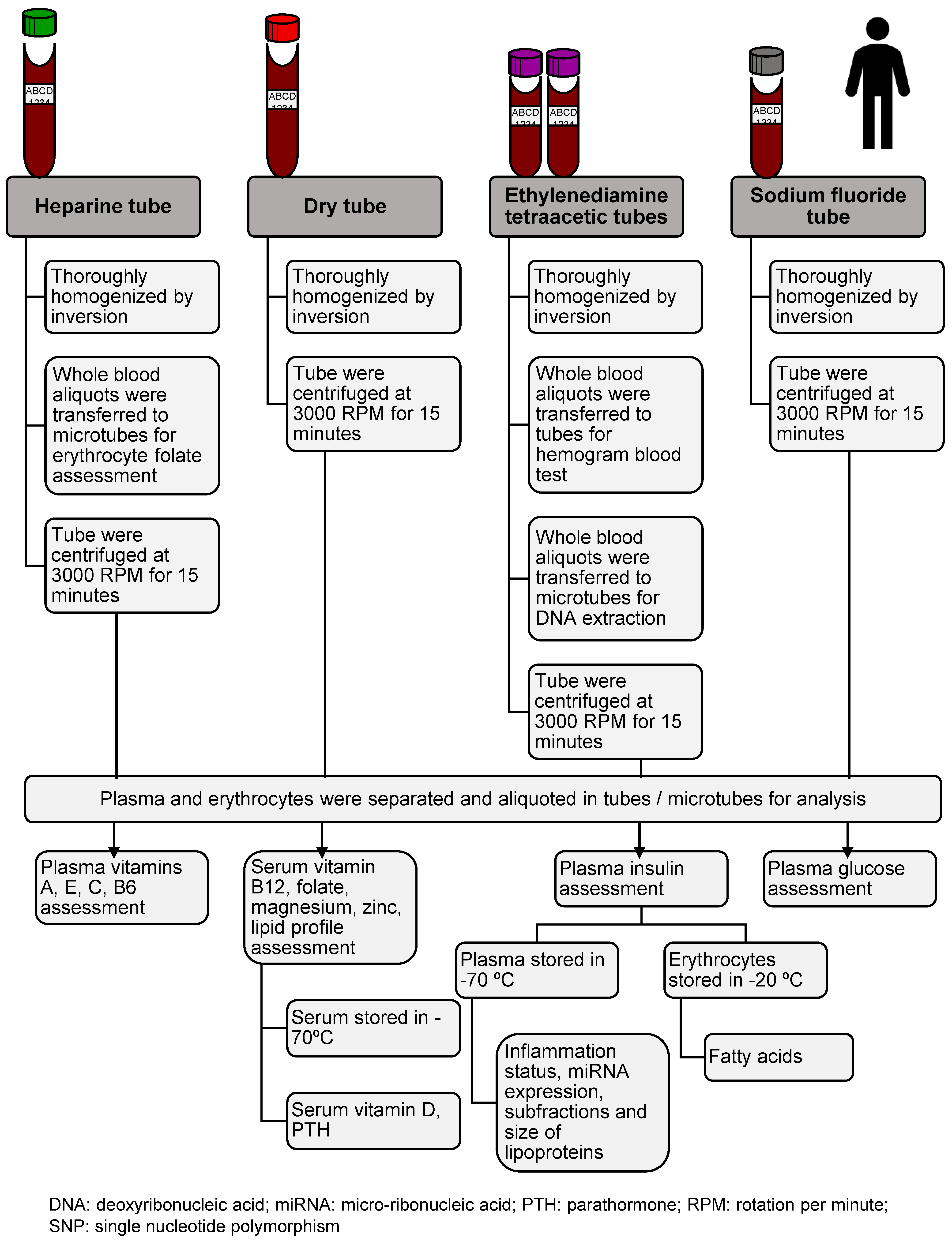
| Analyte | Sample | Technical Reference |
|---|---|---|
| Vitamin A (retinol) | Heparinized plasma | High performance liquid chromatography method (Ref. # KC 1600; Immundiagnostik AG, Bensheim, HE, Germany) |
| Vitamin C (ascorbic acid) | Heparinized plasma | High performance liquid chromatography method (Ref. # KC 2900; Immundiagnostik AG, Bensheim, HE, Germany) |
| Vitamin E (alpha-tocopherol) | Heparinized plasma | High performance liquid chromatography method (Ref. # KC 1600; Immundiagnostik AG, Bensheim, HE, Germany) |
| Vitamin D (cholecalciferol-25 (OH)D3) | Serum | Chemiluminescence (Diasorin kit, Stillwater, MN, USA) |
| Vitamin B6 (pyridoxal-5-phosphate) | Heparinized plasma | High performance liquid chromatography method (Ref. # KC 2100; Immundiagnostik AG, Bensheim, HE, Germany) |
| Vitamin B9 (folate) | Serum and erythrocytes | Chemiluminescent immunoassay (Ref. # A98032; Beckman Coulter Inc.; Fullerton, CA, USA) |
| Vitamin B12 (cobalamin) | Serum | Chemiluminescent immunoassay (Ref. # 3300; Beckman Coulter Inc.; Fullerton, CA, USA) |
| Parathormone | Serum | Elecsys 2010 kit, Roche Diagnostics, Indianapolis, ID, USA |
| Iron | Serum | Colorimetric assay (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany) |
| Ferritin | Serum | Chemiluminescent immunoassay (Ref. # 33020; Beckman Coulter Inc.; Fullerton, CA, USA) |
| Transferrin | Serum | Kinetic nephelometry (Ref. # OSR6152; Beckman Coulter Inc.; Fullerton, CA, USA) |
| Whole blood cell count | Whole blood | Automatic method |
| Magnesium | Serum | Photometric color assay (Ref. # OSR6189; Beckman Coulter Inc.; Fullerton, CA, USA) |
| Zinc | Serum | Flame atomic absorption method (AAnalyst 100 instrument; Perkin Elmer, Norwalk, CT, USA) [10] |
| Total cholesterol | Serum | Trinder reaction (cholesterol oxidase) (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany) |
| Low-density lipoprotein-cholesterol | Serum | Homogeneous enzymatic colorimetric assay (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany) |
| High-density lipoprotein-cholesterol | Serum | Homogeneous enzymatic colorimetric assay (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany) |
| Triacylglycerol | Serum | Enzymatic colorimetric assay (glycerol phosphate peroxidase) (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany) |
| Very low-density lipoprotein-cholesterol | Serum | Values were calculated by dividing the triglyceride values by five |
| Non-high-density lipoprotein-cholesterol | Serum | Values were determined as the difference between the values of total cholesterol and high-density lipoprotein-cholesterol |
| Glucose | Sodium fluoride plasma | Colorimetric enzymatic assay of glucose oxidase (Trinder reaction) (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany) |
| Insulin | Ethylenediamine tetraacetic plasma | Multiplex immunoassay (LINCOplex®; Linco Research Inc., St. Charles, MO, USA) |
| Variables | First 24HR (In-Person) | Second 24HR (Phone) | Both 24HR |
|---|---|---|---|
| Total (n) | 1744 | 548 | 2292 |
| Age category (%) a | |||
| Adolescent (12 to 19 years) | 31.8 | 33.0 | 32.1 |
| Adult (20 to 59 years) | 36.9 | 28.8 | 35.0 |
| Older adult (≥60 years) | 31.3 | 38.1 | 32.9 |
| São Paulo geographical area for health assistance (%) | |||
| North | 14.8 | 13.5 | 14.5 |
| Midwest | 17.6 | 20.4 | 18.3 |
| Southeast | 22.5 | 23.4 | 22.7 |
| South | 26.3 | 25.2 | 26.0 |
| East | 18.9 | 17.5 | 18.6 |
| Weekend; Saturday, Sunday (%) | 31.1 | 19.3 | 28.3 |
| Intake reliability (% Reliable) b | 94.0 | 91.4 | 93.4 |
| Intake amount (%) | |||
| Close to the amount usually eaten | 84.8 | 75.2 | 82.5 |
| A lot more than usually eaten | 5.8 | 8.2 | 6.4 |
| A lot less than usually eaten | 9.4 | 16.6 | 11.1 |
| Energy intake (%) | |||
| Less than 800 kcal | 5.4 | 6.0 | 5.6 |
| More than 4000 kcal | 2.8 | 2.0 | 2.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisberg, R.M.; Sales, C.H.; Fontanelli, M.D.M.; Pereira, J.L.; Alves, M.C.G.P.; Escuder, M.M.L.; César, C.L.G.; Goldbaum, M. 2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures. Nutrients 2018, 10, 169. https://doi.org/10.3390/nu10020169
Fisberg RM, Sales CH, Fontanelli MDM, Pereira JL, Alves MCGP, Escuder MML, César CLG, Goldbaum M. 2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures. Nutrients. 2018; 10(2):169. https://doi.org/10.3390/nu10020169
Chicago/Turabian StyleFisberg, Regina Mara, Cristiane Hermes Sales, Mariane De Mello Fontanelli, Jaqueline Lopes Pereira, Maria Cecília Goi Porto Alves, Maria Mercedes Loureiro Escuder, Chester Luís Galvão César, and Moisés Goldbaum. 2018. "2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures" Nutrients 10, no. 2: 169. https://doi.org/10.3390/nu10020169






