Macronutrient Composition and Food Form Affect Glucose and Insulin Responses in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Test Foods
2.4. Measurement of GR and IR and Determination of GI and II
2.5. Nutrient Composition
2.6. Statistical Analysis
3. Results
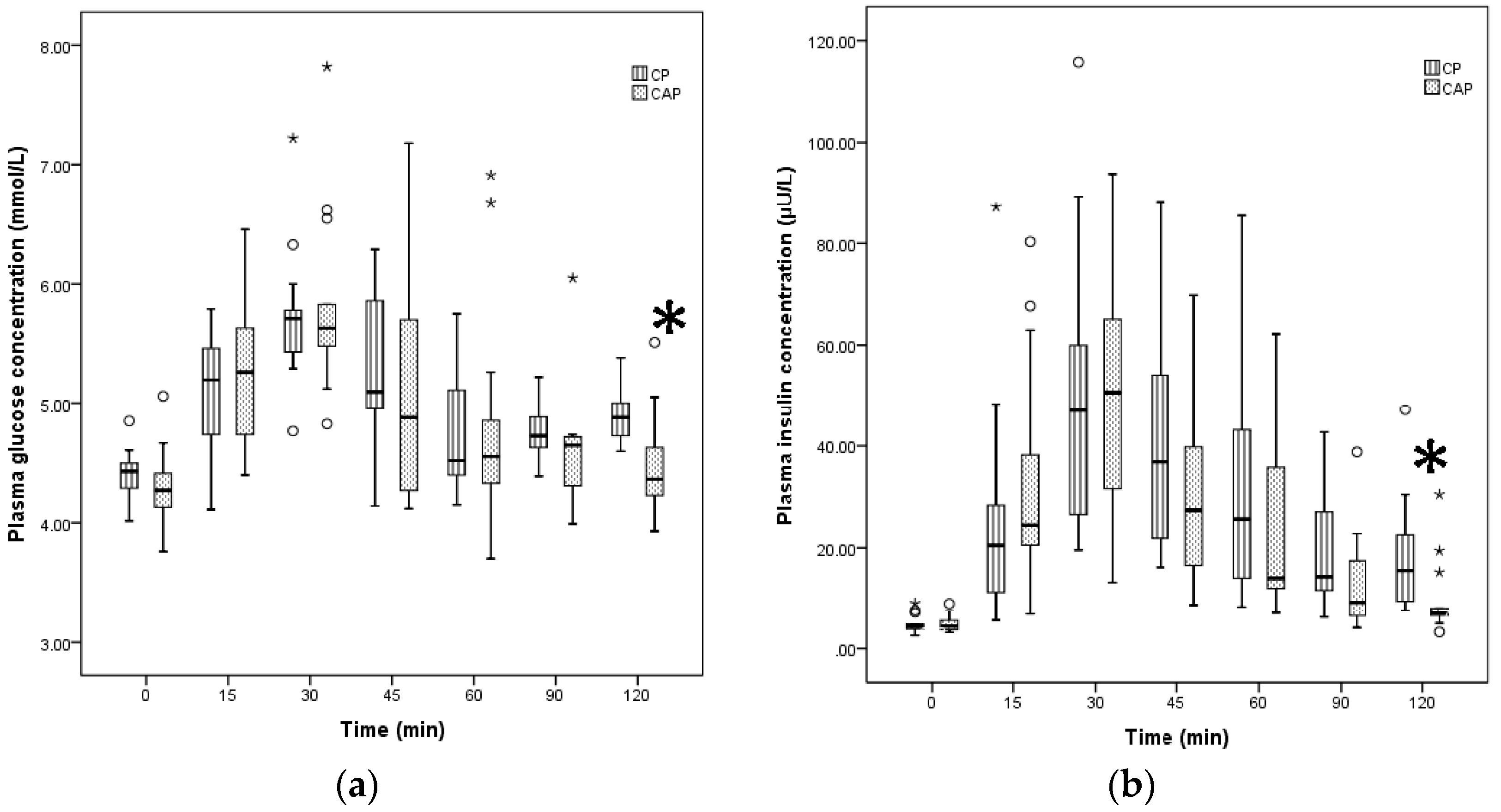
3.1. Comparison of GI, II, GR, and IR for Two Liquid Foods
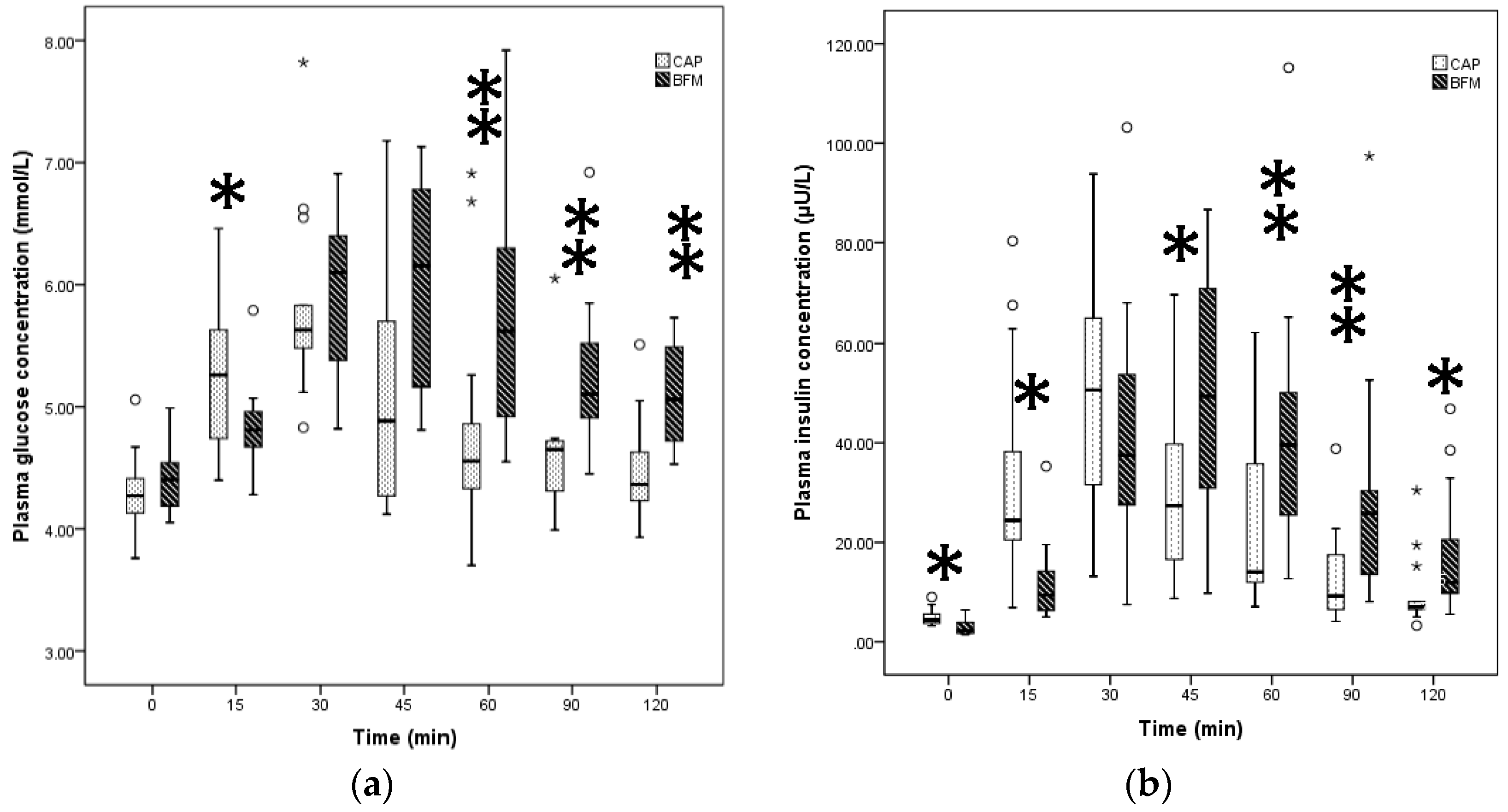
3.2. Comparison of the GI, II, GR, and IR for Liquid vs. Solid Foods
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization (FAO); World Health Organisation (WHO). Carbohydrates in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Pap. 1998, 66, 1–140. [Google Scholar]
- Sentko, A. Innovative low-glycaemic carbohydrates: An update. Nutrafoods 2013, 12, 127–135. [Google Scholar] [CrossRef]
- Howard, B.V.; Wylie-Rosett, J. Sugar and cardiovascular disease: A statement for healthcare professionals from the committee on nutrition of the council on nutrition, physical activity, and metabolism of the American Heart Association. Circulation 2002, 106, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H. Dietary guidelines for sugar: The need for evidence. Br. J. Nutr. 2003, 90, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Bornet, F.R.; Alamowitch, C.; Slama, G. Methods to assess glucose metabolism in humans in relation to carbohydrate and fibre in the diet. Eur. J. Clin. Nutr. 1995, 49, S97–S104. [Google Scholar] [PubMed]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Erbe, J.K. Low glycemic index diets for the management of diabetes. Am. Fam. Phys. 2009, 80, 941. [Google Scholar]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Majzoub, J.A.; Al-Zahrani, A.; Dallal, G.E.; Blanco, I.; Roberts, S.B. High glycemic index foods, overeating, and obesity. Pediatrics 1999, 103, E26. [Google Scholar] [CrossRef] [PubMed]
- Alfenas, R.C.; Mattes, R.D. Influence of glycemic index/load on glycemic response, appetite, and food intake in healthy humans. Diabetes Care 2005, 28, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.D.; Keller, K.R.; Moyer-Mileur, L.J.; Ding, Y.W.; Donaldson, D.; Jackson, W.D. Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents. Pediatrics 2003, 111, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Edes, T.E.; Shah, J.H. Glycemic index and insulin response to a liquid nutritional formula compared with a standard meal. J. Am. Coll. Nutr. 1998, 17, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M. Glycemic index claims on food labels: Review of Health Canada’s evaluation. Eur. J. Clin. Nutr. 2013, 67, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Dumais, L.; Barber, J. Health Canada’s evaluation of the use of glycemic index claims on food labels. Am. J. Clin. Nutr. 2013, 98, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hoefkens, C.; Verbeke, W. Consumers’ health-related motive orientations and reactions to claims about dietary calcium. Nutrients 2013, 5, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buul, J.V.; Brouns, F.J. Nutrition and health claims as marketing tools. Crit. Rev. Food Sci. Nutr. 2015, 55, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Zhu, W.; Fan, F.; Bai, Q.; Huang, C.; Liu, J.; Li, Z.; Sederholm, M.; Norstedt, G.; et al. Effect of a macronutrient preload on blood glucose level and pregnancy outcome in gestational diabetes. J. Clin. Transl. Endocrinol. 2016, 16, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Van Helvoort, A.; Henry, C.J. Glycaemic responses to liquid food supplements among three Asian ethnic groups. Eur. J. Nutr. 2016, 55, 2493–2498. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.B.; Pasin, G. Emerging evidence for the importance of dietary protein source on glucoregulatory markers and type 2 diabetes: Different effects of dairy, meat, fish, egg, and plant protein foods. Nutrients 2016, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.H.; Miller, J.C.; Petocz, P. An insulin index of foods: The insulin demand generated by 1000-kJ portions of common foods. Am. J. Clin. Nutr. 1997, 66, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Venn, B.J.; Green, T.J. Glycemic index and glycemic load: Measurement issues and their effect on diet-disease relationships. Eur. J. Clin. Nutr. 2007, 61, S122–S131. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Atkinson, F.; Rowan, A. Effect of added carbohydrates on glycemic and insulin responses to children’s milk products. Nutrients 2013, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, P.; Guo, H.; Ling, W. Taking a low glycemic index multi-nutrient supplement as breakfast improves glycemic control in patients with type 2 diabetes mellitus: A randomized controlled trial. Nutrients 2014, 6, 5740–5755. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Atkinson, F.S.; Ramalingam, N.; Buyken, A.E.; Brand-Miller, J.C. Effects of human milk and formula on postprandial glycaemia and insulinaemia. Eur. J. Clin. Nutr. 2015, 69, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar]
- Björck, I.; Liljeberg, H.; Ostman, E. Low glycaemic-index foods. Br. J. Nutr. 2000, 83, S149–S155. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.; Bjorck, I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.G.; Akerberg, A.K.; Bjorck, I.M. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 647–655. [Google Scholar] [PubMed]
- Nuttall, F.Q.; Gannon, M.C. Metabolic response of people with type 2 diabetes to a high protein diet. Nutr. Metab. 2004, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.M.; Heden, T.D.; Liu, Y.; Nyhoff, L.M.; Thyfault, J.P.; Leidy, H.J.; Kanaley, J.A. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J. Nutr. 2015, 145, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Liu, V.; Petocz, P.; Baxter, R.C. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am. J. Clin. Nutr. 2005, 82, 350–354. [Google Scholar] [PubMed]
- Gunnerud, U.J.; Ostman, E.M.; Bjorck, I.M. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur. J. Clin. Nutr. 2013, 67, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Mooradian, A.D.; Gannon, M.C.; Billington, C.; Krezowski, P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984, 7, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Bjorck, I.M. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Effects of differences in amount and kind of dietary carbohydrate on plasma glucose and insulin responses in man. Am. J. Clin. Nutr. 1979, 32, 2568–2578. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Janle, E.; Campbell, W.W. Postprandial glycemic and insulinemic responses to common breakfast beverages consumed with a standard meal in adults who are overweight and obese. Nutrients 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Schouten, L.J.; Jurgens, J.; Endert, E.; Kalsbeek, A.; Fliers, E.; Bisschop, P.H. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2014, 112, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Light, K.; Henderson, M.; O’Loughlin, J.; Mathieu, M.E.; Paradis, G.; Gray-Donald, K. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J. Nutr. 2014, 144, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Apolzan, J.W.; Leidy, H.J.; Mattes, R.D.; Campbell, W.W. Effects of food form on food intake and postprandial appetite sensations, glucose and endocrine responses, and energy expenditure in resistance trained v. sedentary older adults. Br. J. Nutr. 2011, 106, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Castellanos, V.H.; Pelkman, C.L.; Thorwart, M.L.; Rolls, B.J. Energy density of foods affects energy intake in normal-weight women. Am. J. Clin. Nutr. 1998, 67, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, K.R.; Purhonen, A.K.; Salmenkallio-Marttila, M.; Lahteenmaki, L.; Laaksonen, D.E.; Herzig, K.H.; Uusitupa, M.I.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Gowland, P.A.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P.; Fillery-Travis, A.J. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1227–G1233. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.C.; Markovic, T.P.; Ross, G.P.; Foote, D.; Brand-Miller, J.C. Timing of peak blood glucose after breakfast meals of different glycemic index in women with gestational diabetes. Nutrients 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Edelbroek, M.A.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The international federation of gynecology and obstetrics (FIGO) initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131, S173–S211. [Google Scholar] [CrossRef]
| Test Food 1 | Food Form 2 | Total Calorie (kcal/100 g or mL) | CHO (g and %) 3 | CHO Type | Protein (g) 3 | Protein Type | Fat (g) 3 | Fiber (g) | Daily Serving Size (mL) |
|---|---|---|---|---|---|---|---|---|---|
| RF | 50 g + 250 mL water | 200 | 50 (100%) | Dextrose anhydrous | - | - | - | - | - |
| CP | 427 mL beverage | 444 (104) | 50 (45%) | Lactose Isomaltulose Slowly digestible starch | 20.9 (18.8%) | Soy Whey casein | 16.2 (32.8%) | 8.5 | 200 |
| CAP | 86.2 g powder + 346 mL water | 322 (374) | 50 (62.1%) | Lactose Sucrose | 21.5 (26.7%) | Whey casein | 3.9 (11%) | 0 | 230 |
| BFM | 85 g wholemeal bread + 260 mL skim milk + 35 g boiled egg | 356 (356) | 50 (56.2%) | Starch Lactose | 23.4 (26.3%) | Gluten Whey casein | 6.4 (16.20%) | 5.1 |
| Test Foods | Peak Glucose (mmol/L) | AUCG (mmol /L) | GI | Peak Insulin (µU/L) | AUCI (µU/L) | II |
|---|---|---|---|---|---|---|
| Reference food | 7.62 (1.23) | 203.38 (68.11) | 100 | 52.13 (34.48) | 2881.03 (3401.76) | 100 |
| CP | 5.70 (0.43) | 68.65 (38.18) | 29.50 (8.00) | 47.05 (40.79) | 2189.7 (1514.75) | 71.50 (27.00) |
| CAP | 5.63 (0.58) | 65.50 (61.05) a | 25.50 (19.75) a | 50.66 (35.93) | 1781.6 (1817.00) a | 52.50 (40.25) a |
| BFM | 6.15 (1.68) | 117.50 (55.53) b | 54.50 (27.50) b | 49.37 (41.48) | 3254.35 (2534.83) b | 98 (70.00) b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafaeizadeh, S.; Muhardi, L.; Henry, C.J.; Van de Heijning, B.J.M.; Van der Beek, E.M. Macronutrient Composition and Food Form Affect Glucose and Insulin Responses in Humans. Nutrients 2018, 10, 188. https://doi.org/10.3390/nu10020188
Shafaeizadeh S, Muhardi L, Henry CJ, Van de Heijning BJM, Van der Beek EM. Macronutrient Composition and Food Form Affect Glucose and Insulin Responses in Humans. Nutrients. 2018; 10(2):188. https://doi.org/10.3390/nu10020188
Chicago/Turabian StyleShafaeizadeh, Shila, Leilani Muhardi, Christiani Jeyakumar Henry, Bert J. M. Van de Heijning, and Eline M. Van der Beek. 2018. "Macronutrient Composition and Food Form Affect Glucose and Insulin Responses in Humans" Nutrients 10, no. 2: 188. https://doi.org/10.3390/nu10020188





