Markers of Iron Status Are Associated with Risk of Hyperuricemia among Chinese Adults: Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Data
2.3. Assessment of Dietary Intake
2.4. Assessment of Physical Activity and Other Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CHNS | the China Health and Nutrition Survey |
| CI | confidence interval |
| CRP | C-reactive protein |
| E% | percentage of energy |
| Hb | hemoglobin |
| hs-CRP | high-sensitivity C-reactive protein |
| HU | hyperuricemia |
| IL-6 | interleukin-6 |
| MET | metabolic equivalent |
| NCDs | non-communicable diseases |
| OR | odds ratio |
| Ref. | reference |
| SD | standard deviation |
| SE | standard error |
| SF | serum ferritin |
| sTfR | soluble transferrin receptors |
| SUA | serum uric acid |
| TfR | transferrin receptors |
| TNF-α | tumor necrosis factor alpha |
| WBC | white blood cell count |
| XO | xanthine oxidase |
References
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Ford, E.S.; Kennedy, T.P.; Hoidal, J.R. The association between serum ferritin and uric acid in humans. Free Radic. Res. 2005, 39, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Nakaoka, H.; Yamamoto, K.; Sakiyama, M.; Shaukat, A.; Toyoda, Y.; Okada, Y.; Kamatani, Y.; Nakamura, T.; Takada, T.; et al. Gwas of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann. Rheum. Dis. 2017, 76, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; van der A, D.L.; Spijkerman, A.M.; Schulze, M.B.; van der Schouw, Y.T. Plasma uric acid is associated with increased risk of type 2 diabetes independent of diet and metabolic risk factors. J. Nutr. 2013, 143, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, L.; Song, M.; Song, Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: A meta-analysis of prospective studies. Atherosclerosis 2013, 231, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Song, J.; Wang, L.; Zhang, S.; Wang, Y. Hyperuricemia and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Trifiro, G.; Morabito, P.; Cavagna, L.; Ferrajolo, C.; Pecchioli, S.; Simonetti, M.; Bianchini, E.; Medea, G.; Cricelli, C.; Caputi, A.P.; et al. Epidemiology of gout and hyperuricaemia in italy during the years 2005–2009: A nationwide population-based study. Ann. Rheum. Dis. 2013, 72, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Tang, Z.; Fang, X.; Wu, X.; Liu, H.; Wang, C.; Hou, C. Prevalence of hyperuricemia among Beijing post-menopausal women in 10 years. Arch. Gerontol. Geriatr. 2016, 64, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, F.; Doualla, M.S.; Lekpa, F.K.; Temfack, E.; Nouga, Y.N.; Donfack, O.S.; Dzudie, A.; Kingue, S. Prevalence and factors associated with hyperuricaemia in newly diagnosed and untreated hypertensives in a sub-Saharan african setting. Arch. Cardiovasc. Dis. 2016, 109, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.X.; Xiong, Y.L.; Zeng, C.; Wei, J.; Yang, T.; Li, H.; Wang, Y.L.; Gao, S.G.; Li, Y.S.; Lei, G.H. Association between low dietary zinc and hyperuricaemia in middle-aged and older males in China: A cross-sectional study. BMJ Open 2015, 5, e008637. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Trevisan, C.; Bolzetta, F.; De Rui, M.; Maggi, S.; Sartori, L.; Musacchio, E.; Zambon, S.; Perissinotto, E.; et al. Results of a observational cohort study of Hyperuricemia as a predictor of poor physical performance in the Elderly. Arthritis Care Res. (Hoboken) 2017, 69, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ding, X.; Wang, Y.L.; Zeng, C.; Wei, J.; Li, H.; Xiong, Y.L.; Gao, S.G.; Li, Y.S.; Lei, G.H. Association between high-sensitivity C-reactive protein and hyperuricemia. Rheumatol. Int. 2016, 36, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Voruganti, V.S.; Laston, S.; Haack, K.; Mehta, N.R.; Cole, S.A.; Butte, N.F.; Comuzzie, A.G. Serum uric acid concentrations and SLC2A9 genetic variation in Hispanic children: The Viva La Familia Study. Am. J. Clin. Nutr. 2015, 101, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Flais, J.; Bardou-Jacquet, E.; Deugnier, Y.; Coiffier, G.; Perdriger, A.; Chales, G.; Ropert, M.; Loreal, O.; Guggenbuhl, P. Hyperferritinemia increases the risk of hyperuricemia in HFE-hereditary hemochromatosis. Jt. Bone Spine 2017, 84, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G., III; Knoll, M.E.; Everett, C.J.; Matheson, E.M.; Hulihan, M.M.; Grant, A.M. Uric acid as a potential cue to screen for iron overload. J. Am. Board Fam. Med. 2011, 24, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Xu, C.; Xu, L.; Yu, C.; Miao, M.; Xie, J.; Li, Y. High serum ferritin levels increase the risk of hyperuricemia: A cross-sectional and longitudinal study. Ann. Nutr. Metab. 2014, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Hong, L.; Zhao, Y.; Sun, H.; Li, L. The association between hyperuricemia and hematological indicators in a Chinese adult population. Medicine (Baltimore) 2016, 95, e2822. [Google Scholar] [CrossRef] [PubMed]
- Beyl, R.N., Jr.; Hughes, L.; Morgan, S. Update on importance of diet in gout. Am. J. Med. 2016, 129, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lou, S.; Xu, K.; Meng, Z.; Zhang, Q.; Song, K. Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin. Rheumatol. 2013, 32, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Wei, J.; Zeng, C.; Li, L.J.; Xie, X.; Wang, Y.L.; Xie, D.X.; Li, H.; Yang, C.; et al. Relationship between cigarette smoking and hyperuricemia in middle-aged and elderly population: A cross-sectional study. Rheumatol. Int. 2017, 37, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Feelders, R.A.; Vreugdenhil, G.; Eggermont, A.M.; Kuiper-Kramer, P.A.; van Eijk, H.G.; Swaak, A.J. Regulation of iron metabolism in the acute-phase response: Interferon gamma and tumour necrosis factor alpha induce hypoferraemia, ferritin production and a decrease in circulating transferrin receptors in cancer patients. Eur. J. Clin. Investig. 1998, 28, 520–527. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A. Interpreting indicators of iron status during an acute phase response—Lessons from malaria and human immunodeficiency virus. Ann. Clin. Biochem. 2008, 45, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Asobayire, F.S.; Adou, P.; Davidsson, L.; Cook, J.D.; Hurrell, R.F. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: A study in cote d’ivoire. Am. J. Clin. Nutr. 2001, 74, 776–782. [Google Scholar] [PubMed]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort profile: The China Health and Nutrition Survey—Monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, S.; Xun, P.; Sharma, S.; Wang, H.; Zhai, F.; Popkin, B. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 2011, 93, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Attard, S.M.; Herring, A.H.; Mayer-Davis, E.J.; Popkin, B.M.; Meigs, J.B.; Gordon-Larsen, P. Multilevel examination of diabetes in modernising China: What elements of urbanisation are most associated with diabetes? Diabetologia 2012, 55, 3182–3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, O.M.; Fonseca, V.F.; Borges, I.; Martins, V.; Portal, V.L.; Pellanda, L.C. C-Reactive protein predicts acute myocardial infarction during high-risk noncardiac and vascular surgery. Clinics (Sao Paulo) 2011, 66, 773–776. [Google Scholar] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Chen, R.; Zheng, W.; Guo, C.; Lu, L.; Ji, X.; Chi, Z.; Yu, J. Association between serum magnesium and anemia: China health and nutrition survey. Biol. Trace Elem. Res. 2014, 159, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, J.; Li, S.; Zhang, B.; Du, S.; Gordon-Larsen, P.; Adair, L.; Popkin, B. The expanding burden of cardiometabolic risk in China: The China health and nutrition survey. Obes. Rev. 2012, 13, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Wang, Y.X.; Li, J.; Zhang, X.L.; Bian, C.; Wang, H.; Du, S.; Suo, L.N. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China health and nutrition survey. Mol. Nutr. Food Res. 2014, 58, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhang, J.; Yuan, G.; Zhang, M.; Zhou, X.; Liu, Z.; Sun, X.; Yu, X. Nontraditional risk factors for cardiovascular disease and visceral adiposity index among different body size phenotypes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 100–107. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shen, X.; Fang, A.; Song, J.; Li, H.; Guo, M.; Li, K. Association between predominantly plant-based diets and iron status in Chinese adults: A cross-sectional analysis. Br. J. Nutr. 2016, 116, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Adair, L.; Gordon-Larsen, P.; Zhang, B.; Popkin, B. Environmental, dietary, and behavioral factors distinguish Chinese adults with high waist-to-height ratio with and without inflammation. J. Nutr. 2015, 145, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.J.; Ley, S.H.; Bhupathiraju, S.N.; Li, Y.; Wang, D.D. Associations of dietary, lifestyle, and sociodemographic factors with iron status in Chinese adults: A cross-sectional study in the China health and nutrition survey. Am. J. Clin. Nutr. 2017, 105, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Dietary pattern is associated with obesity in older people in China: Data from China Health and Nutrition Survey (CHNS). Nutrients 2015, 7, 8170–8188. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Assessing dietary quality of older Chinese people using the Chinese Diet Balance Index (DBI). PLoS ONE 2015, 10, e0121618. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhao, J.; Wu, Y.; Wang, H.; Wang, Z.; Wang, Y.; Zhang, B. Temporal trends in dietary macronutrient intakes among adults in rural China from 1991 to 2011: Findings from the CHNS. Nutrients 2017, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition Tables 2004 (Book 2), 2nd ed.; Beijing Medical University Press: Beijing, China, 2005. [Google Scholar]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition Tables (Book 1), 2nd ed.; Beijing Medical University Press: Beijing, China, 2009. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and met intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norton, E.C.; Popkin, B.M. Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China health and nutrition surveys. Soc. Sci. Med. 2009, 68, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Shi, Z.; Yuan, B.; Dai, Y.; Hu, G.; Wu, G.; Hussain, A. Interaction between physical activity and sleep duration in relation to insulin resistance among non-diabetic Chinese adults. BMC Public Health 2012, 12, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, W.L. Centers for Disease Control and Prevention (CDC); American Heart Association (AHA). CDC/AHA workshop on markers of inflammation and cardiovascular disease: Application to clinical and public health practice: Laboratory tests available to assess inflammation—Performance and standardization: A background paper. Circulation 2004, 110, e572–e576. [Google Scholar] [PubMed]
- Ting, K.; Gill, T.K.; Keen, H.; Tucker, G.R.; Hill, C.L. Prevalence and associations of gout and hyperuricaemia: Results from an Australian population-based study. Intern. Med. J. 2016, 46, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Leon, J.; Denova-Gutierrez, E.; Castanon-Robles, S.; Granados-Garcia, V.; Talavera, J.O.; Rivera-Paredez, B.; Huitron-Bravo, G.G.; Cervantes-Rodriguez, M.; Quiterio-Trenado, M.; Rudolph, S.E.; et al. Sweetened beverage consumption and the risk of hyperuricemia in Mexican adults: A cross-sectional study. BMC Public Health 2014, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Iseki, K.; Inoue, T.; Touma, T.; Ikemiya, Y.; Takishita, S. Hyperuricemia and cardiovascular risk factor clustering in a screened cohort in Okinawa, Japan. Hypertens. Res. 2004, 27, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. Biomed. Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lou, S.; Meng, Z.; Ren, X. Gender and age impacts on the correlations between hyperuricemia and metabolic syndrome in Chinese. Clin. Rheumatol. 2011, 30, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Tenderenda, E.; Taranta-Janusz, K.; Tobolczyk, J.; Stypulkowska, J. Markers of systemic inflammation in children with hyperuricemia. Acta Paediatr. 2012, 101, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Zykova, S.N.; Storhaug, H.M.; Toft, I.; Chadban, S.J.; Jenssen, T.G.; White, S.L. Cross-sectional analysis of nutrition and serum uric acid in two Caucasian cohorts: The ausdiab study and the tromso study. Nutr. J. 2015, 14, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Obi, Y.; Streja, E.; Rhee, C.M.; Catabay, C.J.; Vaziri, N.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Hsu, F.C.; Serra, M.C.; Yank, V.; Pahor, M.; Nicklas, B.J. The effects of a long-term physical activity intervention on serum uric acid in older adults at risk for physical disability. J. Aging Phys. Act. 2014, 22, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ortegon, M.F.; McLachlan, S.; Wild, S.H.; Fernandez-Real, J.M.; Hayward, C.; Polasek, O. Soluble transferrin receptor levels are positively associated with insulin resistance but not with the metabolic syndrome or its individual components. Br. J. Nutr. 2016, 116, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.G.; Gardner, J.E. The relation of serum uric acid to haemoglobin level in patients with cardiac and respiratory disease. J. Clin. Pathol. 1960, 13, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Sevanian, A.; Muakkassah-Kelly, S.F.; Hochstein, P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem. J. 1986, 235, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Muraoka, S.; Ogiso, T. Inhibitory effect of urate on oxidative damage induced by adriamycin-Fe3+ in the presence of H2O2. Res. Commun. Chem. Pathol. Pharmacol. 1993, 79, 75–85. [Google Scholar] [PubMed]
- Furth-Walker, D.; Amy, N.K. Regulation of xanthine oxidase activity and immunologically detectable protein in rats in response to dietary protein and iron. J. Nutr. 1987, 117, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Martelin, E.; Lapatto, R.; Raivio, K.O. Regulation of xanthine oxidoreductase by intracellular iron. Am. J. Physiol. Cell Physiol. 2002, 283, C1722–C1728. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kennedy, T.P.; Stonehuerner, J.; Carter, J.D.; Skinner, K.A.; Parks, D.A.; Hoidal, J.R. Iron regulates xanthine oxidase activity in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L563–L572. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, K.D.; Huecksteadt, T.P.; Hoidal, J.R. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J. Immunol. 1994, 153, 1789–1797. [Google Scholar] [PubMed]
- Jehn, M.; Clark, J.M.; Guallar, E. Serum ferritin and risk of the metabolic syndrome in U.S. Adults. Diabetes Care 2004, 27, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, Y.; Kim, Y.I. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the south Korean general population according to the Korean national health and nutrition examination survey 2008. Metabolism 2011, 60, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cogswell, M.E. Diabetes and serum ferritin concentration among U.S. Adults. Diabetes Care 1999, 22, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.S. Effect of phlebotomy on plasma glucose and insulin concentrations. Diabetes Care 1998, 21, 2190. [Google Scholar] [CrossRef] [PubMed]
- Modan, M.; Halkin, H.; Karasik, A.; Lusky, A. Elevated serum uric acid—A facet of hyperinsulinaemia. Diabetologia 1987, 30, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fu, Q.; Zhang, Q.; Sun, M.; Gao, Y.; Liu, X.; Qian, L.; Shan, S.; Yang, T. The association between serum uric acid and residual β-cell function in type 2 diabetes. J. Diabetes Res. 2014, 2014, 709691. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.; Chen, Y.D.; Hollenbeck, C.B.; Reaven, G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991, 266, 3008–3011. [Google Scholar] [CrossRef] [PubMed]
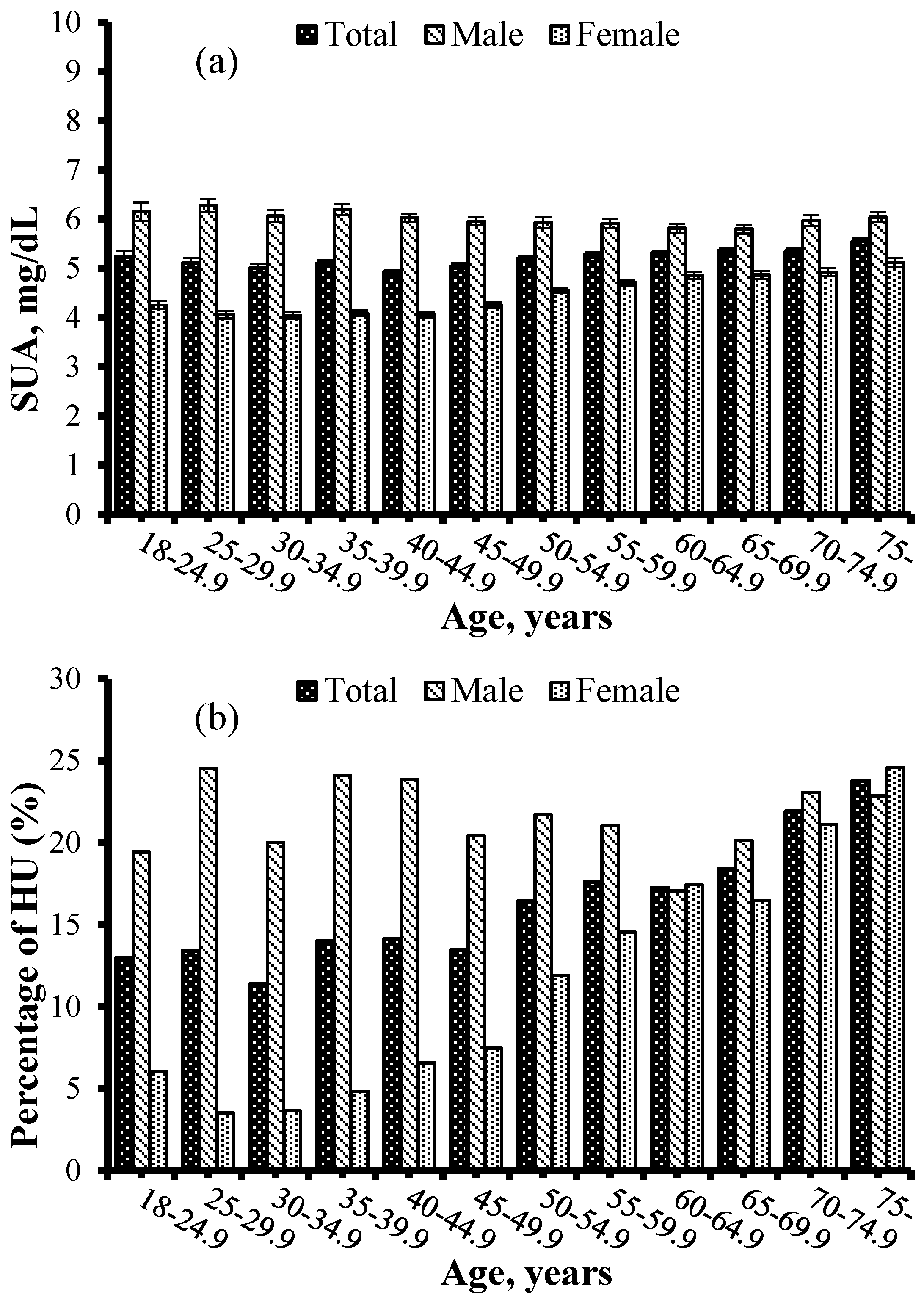
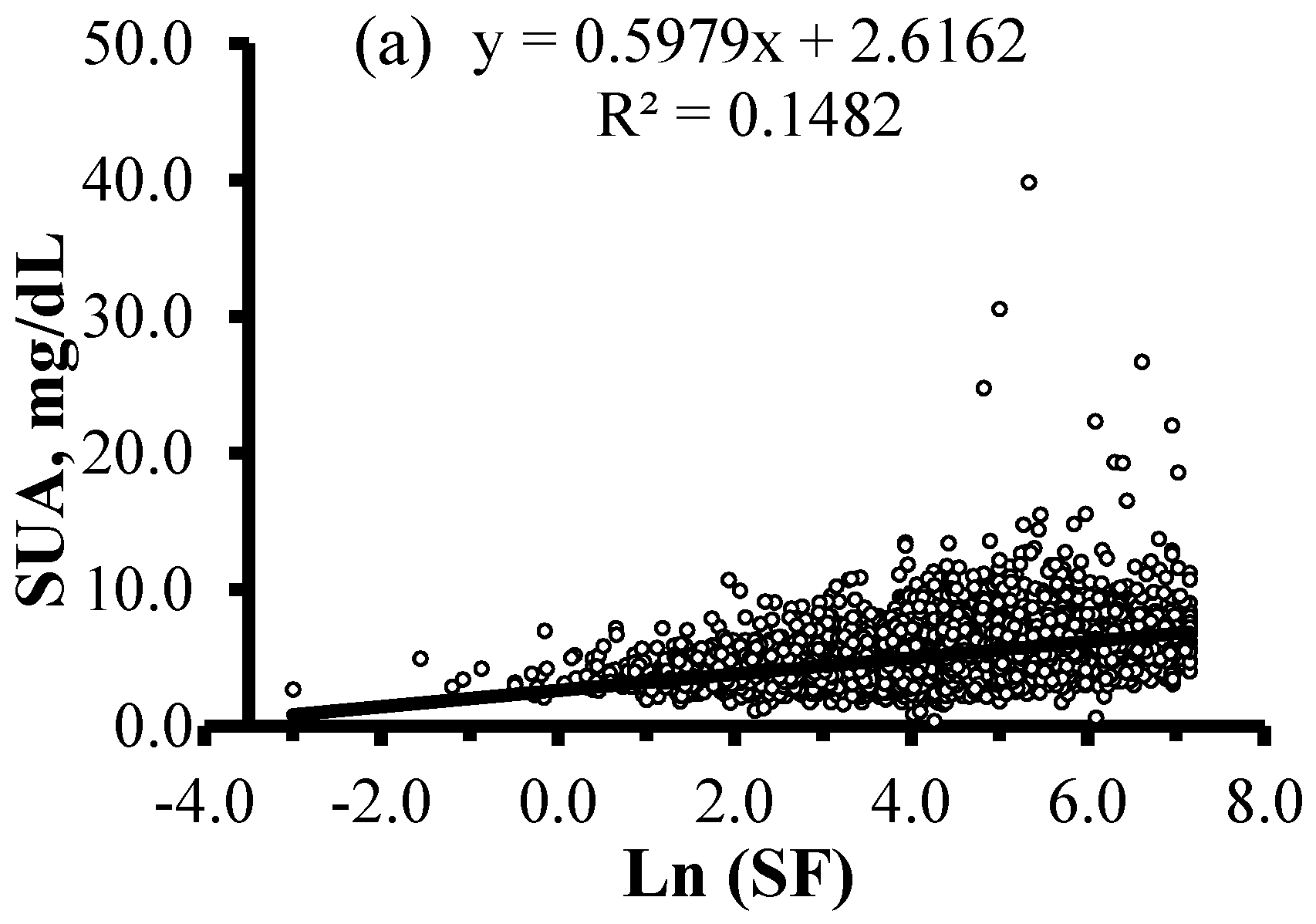
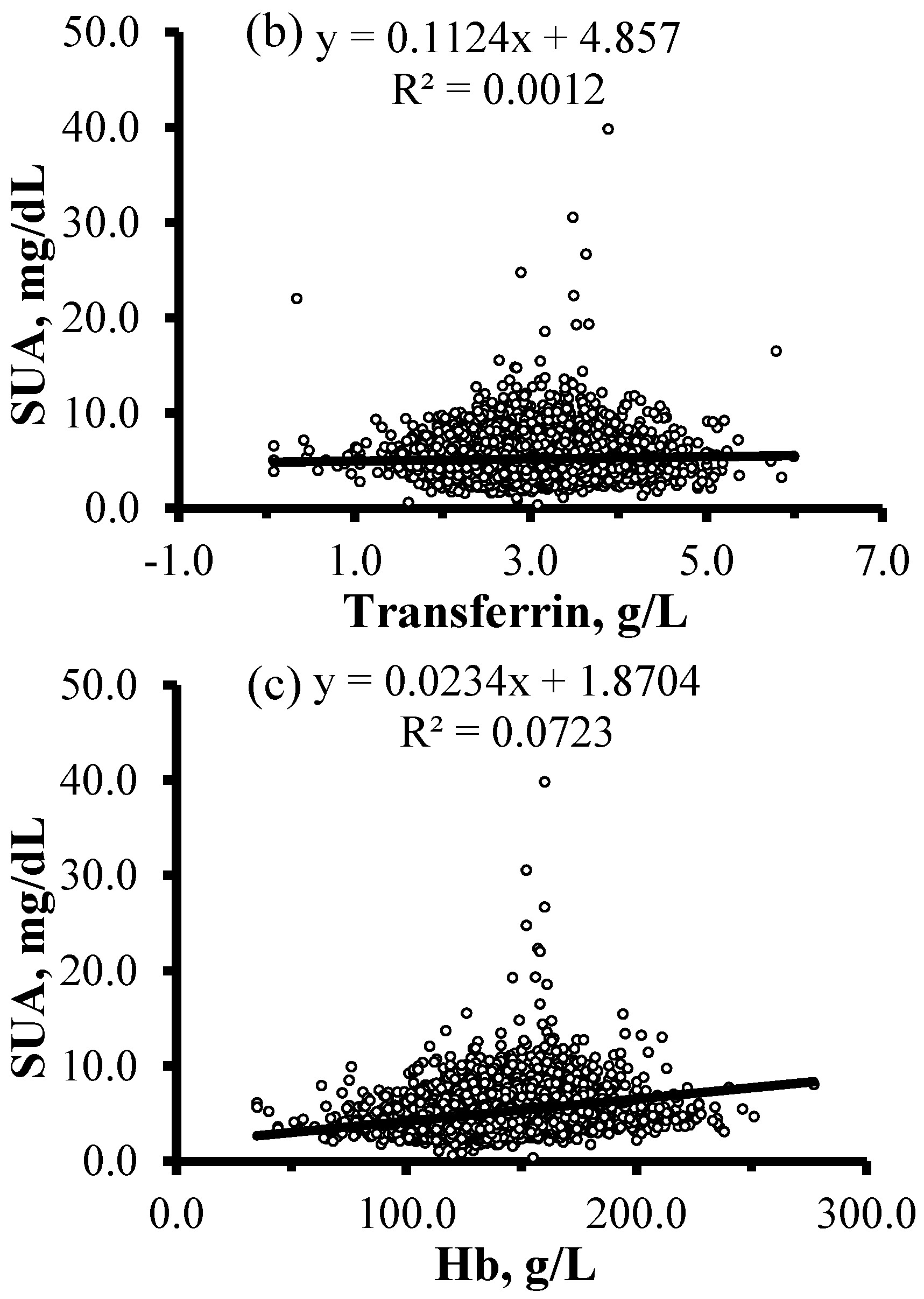
| Characteristics | HU (n = 1276) | Non-HU (n = 6670) | Total (n = 7946) | p |
|---|---|---|---|---|
| SUA, mg/dL | 7.9 ± 2.1 | 4.7 ± 1.1 | 5.2 ± 1.8 | <0.001 |
| Rural area | <0.001 | |||
| Yes | 785 (61.5) | 4546 (68.2) | 5331 (67.1) | |
| No | 491 (38.5) | 2124 (31.8) | 2615 (32.9) | |
| Age, years | 53.4 ± 15.2 | 50.3 ± 14.8 | 50.8 ± 14.9 | <0.001 |
| Gender | <0.001 | |||
| Male | 793 (62.1) | 2917 (43.7) | 3710 (46.7) | |
| Female | 483 (37.9) | 3753 (56.3) | 4236 (53.3) | |
| Nationality | 0.205 | |||
| Han | 1123 (88.0) | 5951 (89.2) | 7074 (89.0) | |
| Others | 153 (12.0) | 719 (10.8) | 872 (11.0) | |
| Education, years | 9 (5, 12) | 9 (5, 9) | 9 (5, 9) | 0.032 |
| BMI, kg/m2 | 24.8 ± 3.6 | 23.1 ± 3.4 | 23.4 ± 3.5 | <0.001 |
| Current smoker | <0.001 | |||
| Yes | 438 (34.3) | 1759 (26.4) | 2197 (27.6) | |
| No | 838 (65.7) | 4911 (73.6) | 5749 (72.4) | |
| Alcohol consumption | <0.001 | |||
| Yes | 560 (43.9) | 2047 (30.7) | 2607 (32.8) | |
| No | 716 (56.1) | 4623 (69.3) | 5339 (67.2) | |
| Physical activities, MET-h/week | 73.6 (31.5, 147.4) | 87.3 (37.0, 182.0) | 84.5 (35.9, 177.0) | <0.001 |
| Total energy intake, kcal/day | 2167.4 (1772.0, 2580.1) | 2063.1 (1669.1, 2499.2) | 2081.6 (1680.7, 2512.3) | <0.001 |
| Carbohydrate intake, g/day | 281.4 (219.3, 354.2) | 282.4 (224.2, 354.8) | 282.3 (223.4, 354.7) | 0.860 |
| Carbohydrate (E%) | 54.2 ± 10.7 | 56.3 ± 10.7 | 55.9 ± 10.7 | <0.001 |
| Protein intake, g/day | 66.0 (52.9, 82.3) | 61.9 (49.6, 77.8) | 62.6 (50.1, 78.6) | <0.001 |
| Protein (E%) | 12.9 ± 3.0 | 12.6 ± 2.9 | 12.6 ± 2.9 | <0.001 |
| Fat intake, g/day | 75.7 (54.2, 99.2) | 68.3 (48.7, 93.1) | 69.5 (49.5, 94.4) | <0.001 |
| Fat (E%) | 32.9 ± 10.8 | 31.2 ± 10.7 | 31.4 ± 10.7 | <0.001 |
| SF, μg/L | 119.3 (71.0, 212.4) | 71.1 (34.9, 131.0) | 78.0 (39.4, 143.3) | <0.001 |
| Transferrin, g/L | 3.0 ± 0.6 | 2.9 ± 0.5 | 2.9 ± 0.5 | <0.001 |
| sTfR, mg/L | 1.33 (1.07, 1.68) | 1.34 (1.09, 1.65) | 1.34 (1.09, 1.65) | 0.407 |
| Hb, g/L | 146.3 ± 20.9 | 140.6 ± 20.3 | 141.5 ± 20.5 | <0.001 |
| Hs-CRP, mg/L | 2.0 (1.0, 3.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | <0.001 |
| SUA, mg/dL | β (95% CI) 1 | Adjust β (95% CI) 2 | Adjust β (95% CI) 3 | ||
|---|---|---|---|---|---|
| SF | Q1 | 4.26 ± 1.25 | Ref. | Ref. | Ref. |
| Q2 | 4.91 ± 1.46 | 0.658 (0.555, 0.761) | 0.310 (0.209, 0.411) | 0.267 (0.167, 0.367) | |
| Q3 | 5.46 ± 1.57 | 1.202 (1.099, 1.305) | 0.587 (0.481, 0.693) | 0.529 (0.423, 0.635) | |
| Q4 | 6.10 ± 2.19 | 1.842 (1.739, 1.945) | 0.991 (0.879, 1.103) | 0.899 (0.788, 1.010) | |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| Transferrin | Q1 | 5.05 ± 1.54 | Ref. | Ref. | Ref. |
| Q2 | 5.15 ± 1.61 | 0.094 (−0.017, 0.205) | 0.196 (0.098, 0.294) | 0.208 (0.112, 0.304) | |
| Q3 | 5.27 ± 1.73 | 0.216 (0.105, 0.327) | 0.334 (0.235, 0.433) | 0.351 (0.254, 0.448) | |
| Q4 | 5.26 ± 2.19 | 0.209 (0.098, 0.320) | 0.457 (0.357, 0.557) | 0.488 (0.389, 0.587) | |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| Hb | Q1 | 4.58 ± 1.50 | Ref. | Ref. | Ref. |
| Q2 | 4.79 ± 1.47 | 0.213 (0.109, 0.317) | 0.046 (−0.051, 0.143) | 0.029 (−0.066, 0.124) | |
| Q3 | 5.45 ± 1.73 | 0.876 (0.769, 0.983) | 0.148 (0.039, 0.257) | 0.114 (0.006, 0.222) | |
| Q4 | 5.98 ± 2.08 | 1.402 (1.294, 1.510) | 0.388 (0.269, 0.507) | 0.353 (0.235, 0.471) | |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 |
| Cases/Total, n | β (95% CI) 1 | Adjust OR (95% CI) 2 | Adjust OR (95% CI) 3 | ||
|---|---|---|---|---|---|
| SF | Q1 | 126/1987 | Ref. | Ref. | Ref. |
| Q2 | 248/1987 | 2.106 (1.684, 2.635) | 1.726 (1.368, 2.179) | 1.618 (1.280, 2.044) | |
| Q3 | 376/1987 | 3.449 (2.790, 4.263) | 2.550 (2.030, 3.203) | 2.344 (1.863, 2.951) | |
| Q4 | 526/1985 | 5.323 (4.332, 6.540) | 3.487 (2.773, 4.384) | 3.086 (2.450, 3.888) | |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| Transferrin | Q1 | 261/2016 | Ref. | Ref. | Ref. |
| Q2 | 307/1991 | 1.226 (1.026, 1.465) | 1.298 (1.080, 1.560) | 1.318 (1.094, 1.587) | |
| Q3 | 333/1953 | 1.383 (1.160, 1.649) | 1.496 (1.246, 1.797) | 1.539 (1.279, 1.852) | |
| Q4 | 375/1986 | 1.565 (1.318, 1.859) | 1.817 (1.514, 2.179) | 1.900 (1.579, 2.286) | |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| Hb | Q1 | 237/1949 | Ref. | Ref. | Ref. |
| Q2 | 277/2182 | 1.050 (0.873, 1.264) | 0.948 (0.784, 1.147) | 0.918 (0.757, 1.113) | |
| Q3 | 336/1924 | 1.528 (1.276, 1.829) | 1.054 (0.862, 1.289) | 0.996 (0.812, 1.221) | |
| Q4 | 426/1891 | 2.100 (1.766, 2.497) | 1.288 (1.044, 1.589) | 1.218 (0.984, 1.507) | |
| p-trend | <0.001 | <0.001 | 0.008 | 0.036 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; He, T.; Yu, K.; Lu, Q.; Alkasir, R.; Guo, G.; Xue, Y. Markers of Iron Status Are Associated with Risk of Hyperuricemia among Chinese Adults: Nationwide Population-Based Study. Nutrients 2018, 10, 191. https://doi.org/10.3390/nu10020191
Li X, He T, Yu K, Lu Q, Alkasir R, Guo G, Xue Y. Markers of Iron Status Are Associated with Risk of Hyperuricemia among Chinese Adults: Nationwide Population-Based Study. Nutrients. 2018; 10(2):191. https://doi.org/10.3390/nu10020191
Chicago/Turabian StyleLi, Xiangping, Tingchao He, Kai Yu, Qian Lu, Rashad Alkasir, Guifang Guo, and Yong Xue. 2018. "Markers of Iron Status Are Associated with Risk of Hyperuricemia among Chinese Adults: Nationwide Population-Based Study" Nutrients 10, no. 2: 191. https://doi.org/10.3390/nu10020191





