Imbalanced Nutrient Intake in Cancer Survivors from the Examination from the Nationwide Health Examination Center-Based Cohort
Abstract
:1. Introduction
2. Materials and Methods
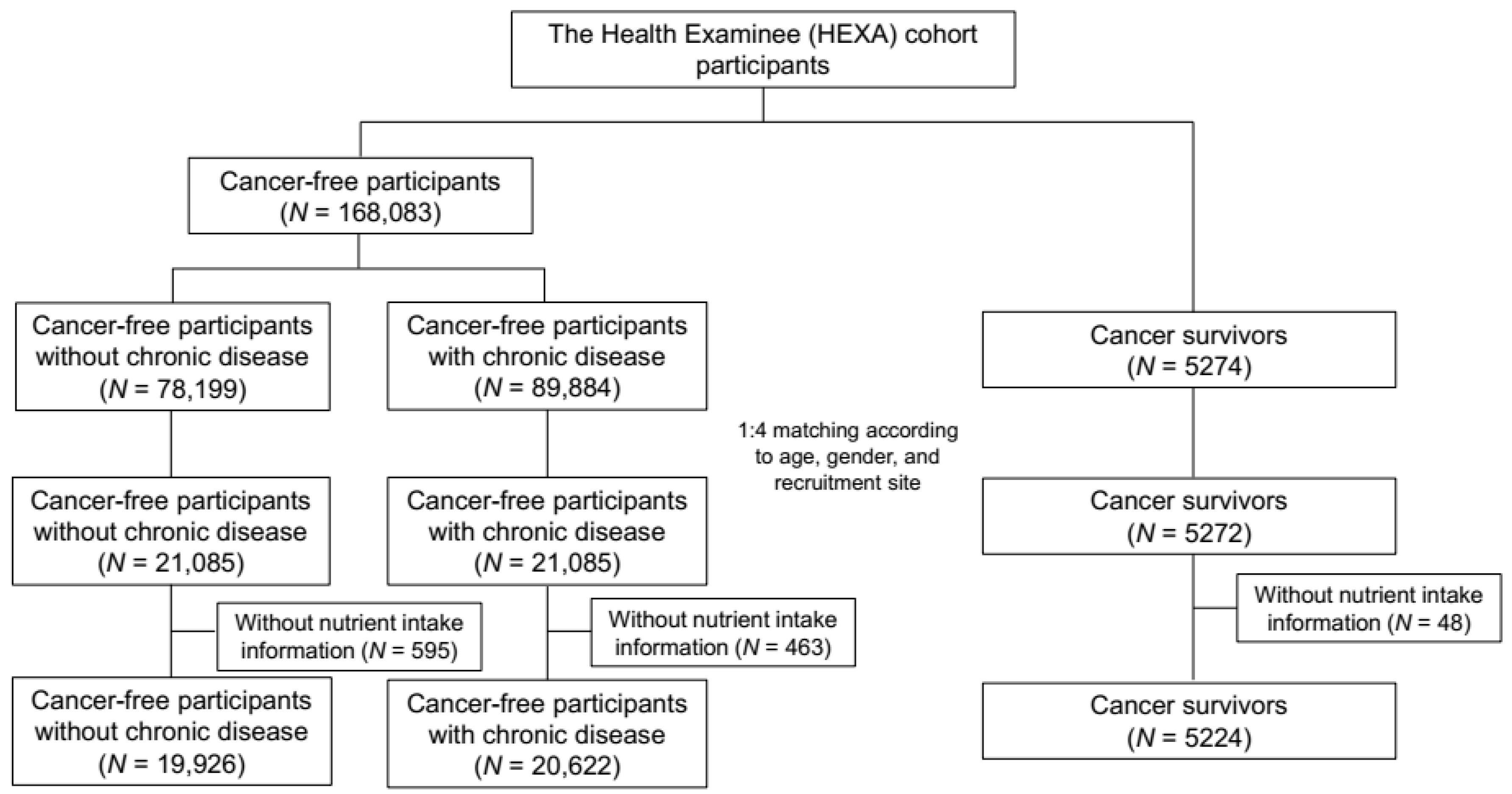
2.1. Data Source and Study Population
2.2. Nutrient Intake
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The global burden of cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- IARC. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 6 June 2016).
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Oh, C.-M.; Cho, H.; Lee, D.H.; Lee, K.H. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2012. Cancer Res. Treat. 2015, 47, 127. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Kalesan, B.; Hoffman, S.C.; Helzlsouer, K.J. Non-cancer adverse health conditions and perceived health and function among cancer survivors participating in a community-based cohort study in Washington County, Maryland. J. Cancer Surviv. 2008, 2, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Eakin, E.G.; Youlden, D.R.; Baade, P.D.; Lawler, S.P.; Reeves, M.M.; Heyworth, J.S.; Fritschi, L. Health status of long-term cancer survivors: Results from an Australian population-based sample. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.B.; Snyder, D.C.; Demark-Wahnefried, W. Lifestyle interventions in cancer survivors: Designing programs that meet the needs of this vulnerable and growing population. J. Nutr. 2007, 137, 243S–248S. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; De Stefani, E.; Ronco, A.; Boffetta, P.; Deneo-Pellegrini, H.; Acosta, G.; Mendilaharsu, M. Meat consumption and cancer risk: A case-control study in Uruguay. Asian Pac. J. Cancer Prev. 2009, 10, 429–436. [Google Scholar] [PubMed]
- Grant, W.B. A multicountry ecological study of cancer incidence rates in 2008 with respect to various risk-modifying factors. Nutrients 2013, 6, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tabung, F.K.; Zhang, J.; Liese, A.D.; Shivappa, N.; Ockene, J.K.; Caan, B.; Kroenke, C.H.; Hebert, J.R.; Steck, S.E. Association between post-cancer diagnosis dietary inflammatory potential and mortality among invasive breast cancer survivors in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Liu, S.; John, E.M.; Must, A.; Demark-Wahnefried, W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer 2015, 121, 4212–4221. [Google Scholar] [CrossRef] [PubMed]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.L.; Currow, D.C. Cancer as a chronic disease. Collegian 2010, 17, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Witter, D.C.; LeBas, J. Cancer as a chronic disease. Oncology 2008, 53, 1–3. [Google Scholar]
- Kim, Y.J.; Go, M.J.; Hu, C.; Hong, C.B.; Kim, Y.K.; Lee, J.Y.; Hwang, J.Y.; Oh, J.H.; Kim, D.J.; Kim, N.H.; et al. Large-scale genome-wide association studies in East Asians identify new genetic loci influencing metabolic traits. Nat. Genet. 2011, 43, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, Y.; Hwang, M.Y.; Shimokawa, K.; Won, S.; Kato, N.; Tabara, Y.; Yokota, M.; Han, B.G.; Lee, J.H.; et al. Identification of a genetic variant at 2q12.1 associated with blood pressure in East Asians by genome-wide scan including gene-environment interactions. BMC Med. Genet. 2014, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Hillard, P.A. Oral contraception noncompliance: The extent of the problem. Adv. Contracept. 1992, 8, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kimm, K.; Park, C.; Kim, D. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, J.E.; Paik, H.Y.; Lee, H.K.; Jo, I.; Kimm, K. Development of a semi-quantitative food frequency questionnaire based on dietary data from the Korea national health and nutrition examination survey. Nutr. Sci. 2003, 6, 173–184. [Google Scholar]
- Ministry of Health and Welfare, The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2015; Ministry of Health and Welfare: Seoul, Korea, 2015.
- World Health Organization Western Pacific Region. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia Pty Limited: Balmain, Australia, 2000. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes—2015 abridged for primary care providers. Clin. Diabetes 2015, 33, 97–111. [Google Scholar]
- Coups, E.J.; Ostroff, J.S. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev. Med. 2005, 40, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Lazovich, D.; Prizment, A.E.; Robien, K. Adherence to the World Cancer Research Fund/American Institute for cancer research recommendations for cancer prevention is associated with better health-related quality of life among elderly female cancer survivors. J. Clin. Oncol. 2013, 31, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Lis, C.G.; Gupta, D.; Lammersfeld, C.A.; Markman, M.; Vashi, P.G. Role of nutritional status in predicting quality of life outcomes in cancer—A systematic review of the epidemiological literature. Nutr. J. 2012, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M. The cardiovascular continuum in Asia—A new paradigm for the metabolic syndrome. J. Cardiovasc. Pharmacol. 2005, 46, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hur, I.; Jang, M.-J.; Oh, K. Food and nutrient intakes according to income in Korean men and women. Osong Public Health Res. Perspect. 2011, 2, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Shim, J.E.; Paik, H.Y.; Song, W.O.; Joung, H. Nutritional intake of Korean population before and after adjusting for within-individual variations: 2001 Korean national health and nutrition survey data. Nutr. Res. Pract. 2011, 5, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Harika, R.K.; Eilander, A.; Alssema, M.; Osendarp, S.J.; Zock, P.L. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann. Nutr. Metab. 2013, 63, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, Z.-C.; Hang, L.-F. Effects of nutritional and psychological status of the patients with advanced stomach cancer on physical performance status. Support. Care Cancer 2009, 17, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.-S. Nutritional status and quality of life of the gastric cancer patients in Changle County of China. World J. Gastroenterol. 2005, 11, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Sulaiman, S.; Koon, P.B.; Amani, R.; Hosseini, S.M. Association of nutritional status with quality of life in breast cancer survivors. Asian Pac. J. Cancer Prev. 2013, 14, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.M.; Emmons, K.M.; Lipkus, I.M. Understanding the potential of teachable moments: The case of smoking cessation. Health Educ. Res. 2003, 18, 156–170. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Peterson, B.; McBride, C.; Lipkus, I.; Clipp, E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 2000, 88, 674–684. [Google Scholar] [CrossRef]
- Gavazzi, C.; Colatruglio, S.; Sironi, A.; Mazzaferro, V.; Miceli, R. Importance of early nutritional screening in patients with gastric cancer. Br. J. Nutr. 2011, 106, 1773. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.Y.; Kim, C.S.; Li, L.; Yang, J.J.; Park, B.; Shin, A.; Chang, S.H.; Lee, K.S.; Kim, H.; Yoo, K.Y.; et al. Validation of self-reported cancer incidence at follow-up in a prospective cohort study. Ann. Epidemiol. 2009, 19, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N (%) | p-Value | ||
|---|---|---|---|---|
| Non-Cancer I (N = 19,926) | Non-Cancer II (N = 20,622) | Cancer Survivors (N = 5224) | ||
| Age | ||||
| Mean ± Standard deviation | 54.6 (8.0) | 55.8 (8.1) | 55.8 (8.1) | <0.001 |
| <50 | 5674 (28.5) | 4851 (23.5) | 1256 (24.0) | <0.001 |
| 50–54 | 4415 (22.2) | 4325 (21.0) | 1098 (21.0) | |
| 55–59 | 3826 (19.2) | 4105 (19.9) | 1014 (19.4) | |
| 60–64 | 3447 (17.3) | 3917 (19.0) | 964 (18.5) | |
| ≥65 | 2564 (12.9) | 3424 (16.6) | 892 (17.1) | |
| Gender | ||||
| Male | 5010 (25.1) | 5169 (25.1) | 1309 (25.1) | 0.98 |
| Female | 14,916 (74.9) | 15,453 (74.9) | 3915 (74.9) | |
| Marital status | ||||
| Married, cohabitant | 17,473 (87.7) | 17,919 (86.9) | 4536 (86.8) | 0.002 |
| Others | 2307 (11.6) | 2617 (12.7) | 671 (12.8) | |
| Missing | 146 (0.7) | 86 (0.4) | 17 (0.3) | |
| Education | ||||
| <High school | 7240 (36.3) | 8266 (40.1) | 1954 (37.4) | <0.001 |
| High school graduate | 7152 (35.9) | 7034 (34.1) | 1870 (35.8) | |
| ≥College | 5174 (26) | 5062 (24.5) | 1344 (25.7) | |
| Missing | 360 (1.8) | 260 (1.3) | 56 (1.1) | |
| Monthly household income | ||||
| <$2000 | 5917 (29.7) | 6697 (32.5) | 1788 (34.2) | <0.001 |
| $2000–3999 | 7210 (36.2) | 6960 (33.8) | 1776 (34.0) | |
| ≥$4000 | 3743 (18.8) | 3769 (18.3) | 949 (18.2) | |
| Missing | 3056 (15.3) | 3196 (15.5) | 711 (13.6) | |
| Employment status | ||||
| Employed | 9547 (47.9) | 8722 (42.3) | 1987 (38) | <0.001 |
| Unemployed | 9891 (49.6) | 11,340 (55) | 3131 (59.9) | |
| Missing | 488 (2.4) | 560 (2.7) | 106 (2.0) | |
| Smoking status | ||||
| Never | 15,898 (79.8) | 16,310 (79.1) | 4112 (78.7) | <0.001 |
| Past | 2171 (10.9) | 2594 (12.6) | 816 (15.6) | |
| Current | 1752 (8.8) | 1643 (8) | 276 (5.3) | |
| Missing | 105 (0.5) | 75 (0.4) | 20 (0.4) | |
| Drinking status | ||||
| Never | 11,267 (56.5) | 11,780 (57.1) | 3254 (62.3) | <0.001 |
| Past | 537 (2.7) | 864 (4.2) | 523 (10.0) | |
| Current | 8006 (40.2) | 7905 (38.3) | 1431 (27.4) | |
| Missing | 116 (0.6) | 73 (0.4) | 16 (0.3) | |
| Regular exercise | ||||
| No | 9662 (48.5) | 9507 (46.1) | 2187 (41.9) | <0.001 |
| <150 min/week | 2976 (14.9) | 3093 (15.0) | 714 (13.7) | |
| ≥150 min/week | 7193 (36.1) | 7979 (38.7) | 2316 (44.3) | |
| Missing | 95 (0.5) | 43 (0.4) | 7 (0.1) | |
| Body mass index | ||||
| <23 kg/m2 | 8799 (44.2) | 7511 (36.4) | 2285 (43.7) | <0.001 |
| 23–24.9 kg/m2 | 5657 (28.4) | 5725 (27.8) | 1384 (26.5) | |
| ≥25 kg/m2 | 5422 (27.2) | 7327 (35.5) | 1537 (29.4) | |
| Missing | 48 (0.2) | 59 (0.3) | 18 (0.3) | |
| Nutrient | Mean (Standard Deviation) | p-Value | ||
|---|---|---|---|---|
| Non-Cancer I (N = 19,926) | Non-Cancer II (N = 20,622) | Cancer Survivors (N = 5224) | ||
| Macronutrients | ||||
| Total energy, kcal | 1739.1 (576.2) | 1713.3 (589.9) a | 1699.2 (559.4) a | <0.001 |
| Protein, g | 59.4 (26.9) | 58.2 (26.9) a | 57.2 (24.7) a,b | <0.001 |
| % Energy | 13.5 (2.7) | 13.4 (2.7) a | 13.3 (2.6) a,b | <0.001 |
| Fat, g | 27.6 (18.3) | 26.5 (18.5) a | 25.0 (16.3) a,b | <0.001 |
| % Energy | 13.8 (5.5) | 13.4 (5.5) a | 12.8 (5.3) a,b | <0.001 |
| Carbohydrate, g | 309.2 (94.4) | 306.5 (97.0) a | 307.8 (95.6) | 0.02 |
| % Energy | 71.7 (7.1) | 72.2 (7.1) a | 73.0 (6.9) a,b | <0.001 |
| Fiber | 5.9 (3.1) | 5.8 (3.1) | 6.0 (3.0) a,b | <0.001 |
| Micronutrients | ||||
| Vitamin A, R.E. | 496.5 (375.2) | 486.0 (373.7) a | 497.0 (353.7) | 0.009 |
| Vitamin B1, mg | 1.0 (0.5) | 1.0 (0.5) a | 1.0 (0.4) a,b | <0.001 |
| Vitamin B2, mg | 0.9 (0.5) | 0.9 (0.5) a | 0.9 (0.4) a | <0.001 |
| Niacin, mg | 14.6 (6.5) | 14.1 (6.5) a | 14.0 (6.0) a | <0.001 |
| Folate, µg | 223.0 (129.5) | 220.7 (131.7) | 227.3 (126.0) b | 0.004 |
| Vitamin B6, mg | 1.6 (0.7) | 1.6 (0.7) a | 1.6 (0.7) | 0.001 |
| Vitamin C, mg | 110.2 (73.0) | 109.2 (74.3) | 114.1 (74.1) a,b | <0.001 |
| Vitamin E, mg | 8.2 (4.7) | 8.1 (4.8) a | 8.3 (4.6) b | 0.009 |
| Minerals | ||||
| Calcium, mg | 458.1 (281.5) | 451.4 (279.9) a | 454.0 (269.1) | 0.049 |
| Phosphorus, mg | 900.4 (378.6) | 883.0 (379.8) a | 876.6 (357.0) a | <0.001 |
| Iron, mg | 10.1 (5.3) | 10.0 (5.3) a | 10.2 (5.2) b | 0.01 |
| Potassium, mg | 2292.3 (1117.5) | 2246.1 (1132.4) a | 2283.6 (1101.9) b | <0.001 |
| Zinc, µg | 8.0 (3.9) | 7.9 (4.1) a | 7.8 (3.6) a | 0.006 |
| Nutrient | Higher Dietary Nutrient Intake Than Recommended Level, N (%) | Odds Ratio a (95% Confidence Interval (CI)) | p-Value b | ||||
|---|---|---|---|---|---|---|---|
| Non-Cancer I (N = 19,926) | Non-Cancer II (N = 20,622) | Cancer Survivors (N = 5224) | Non-Cancer I | Non-Cancer II | Cancer Survivors | ||
| Total energy | 6392 (32.1) | 6438 (31.2) | 1608 (30.8) | Reference | 0.93 (0.89–0.98) | 0.92 (0.86–0.99) | 0.81 |
| Carbohydrate (% of total energy) | 16901 (84.8) | 17649 (85.6) | 4585 (87.8) | 1.01 (0.96–1.08) | 1.21 (1.10–1.33) | <0.001 | |
| Fat (% of total energy) | 203 (1.0) | 184 (0.9) | 26 (0.5) | 0.90 (0.73–1.13) | 0.54 (0.35–0.83) | 0.02 | |
| Protein | 11117 (55.8) | 11046 (53.6) | 2696 (51.6) | 0.90 (0.86–0.94) | 0.85 (0.79–0.90) | 0.05 | |
| Vitamin A | 4429 (22.2) | 4459 (21.6) | 1207 (23.1) | 0.94 (0.89–0.99) | 1.02 (0.95–1.11) | 0.02 | |
| Vitamin B1 | 5871 (29.5) | 5670 (27.5) | 1349 (25.8) | 0.92 (0.88–0.97) | 0.86 (0.80–0.93) | 0.08 | |
| Vitamin B2 | 3395 (17.0) | 3290 (16.0) | 802 (15.4) | 0.94 (0.89–1.00) | 0.89 (0.81–0.97) | 0.22 | |
| Niacin, mg | 8206 (41.2) | 7893 (38.3) | 1976 (37.8) | 0.90 (0.87–0.94) | 0.91 (0.85–0.97) | 0.75 | |
| Folate | 1575 (7.9) | 1535 (7.4) | 441 (8.4) | 0.93 (0.86–1.01) | 1.05 (0.93–1.18) | 0.04 | |
| Vitamin B6 | 10627 (53.3) | 10624 (51.5) | 2775 (53.1) | 0.93 (0.89–0.97) | 1.01 (0.96–1.08) | 0.01 | |
| Vitamin C | 9156 (46.0) | 9347 (45.3) | 2589 (49.6) | 0.98 (0.94–1.03) | 1.16 (1.08–1.23) | <0.001 | |
| Calcium | 2162 (10.9) | 2033 (9.9) | 564 (10.8) | 0.89 (0.83–0.96) | 0.96 (0.87–1.07) | 0.15 | |
| Phosphorus | 13873 (69.6) | 13802 (66.9) | 3434 (65.7) | 0.89 (0.86–0.94) | 0.86 (0.81–0.92) | 0.22 | |
| Iron | 9019 (45.3) | 9579 (46.5) | 2504 (47.9) | 0.98 (0.94–1.03) | 1.05 (0.98–1.13) | 0.045 | |
| Zinc | 8714 (43.7) | 8823 (42.8) | 2233 (42.7) | 0.94 (0.90–0.98) | 0.96 (0.89–1.02) | 0.65 | |
| Nutrient | Mean (Standard Deviation) | ||||||
|---|---|---|---|---|---|---|---|
| Gastric Cancer | Colon Cancer | Breast Cancer | Cervical Cancer | Thyroid Cancer | Other Cancer | Multiple Cancers | |
| N = 727 | N = 372 | N = 864 | N = 603 | N = 880 | N = 1405 | N = 136 | |
| Macronutrients | |||||||
| Total energy, kcal | 1681.3 (552.8) | 1704.1 (531.1) | 1662.1 (55.85) a | 1661.5 (541.4) a | 2734.4 (587.4) | 1726.5 (561.4) | 1628.1 (522.7) |
| Protein, g | 55.9 (25.5) a | 57.3 (24.8) | 55.2 (22.9) a | 56.0 (24.1) a | 58.8 (25.0) | 58.6 (25.5) | 53.9 (22.3) |
| % Energy | 13.1 (2.7) a | 13.3 (2.5) | 13.2 (2.5) | 13.4 (2.7) | 13.4 (2.5) | 13.4 (2.8) | 13.1 (2.4) |
| Fat, g | 24.6 (18.2) a | 24.4 (15.8) a | 22.9 (14.3) a | 24.8 (15.4) a | 26.9 (17.0) | 25.9 (16.4) a | 21.6 (13.2) a |
| % Energy | 12.5 (5.5) a | 12.4 (4.8) a | 12.2 (5.0) a | 13.0 (5.3) a | 13.5 (5.1) | 13.0 (5.5) a | 11.5 (5.1) a |
| Carbohydrate, g | 305.2 (90.9) | 310.2 (88.8) | 306.0 (100.9) | 300.4 (93.7) | 310.8 (98.5) | 311.0 (95.4) | 301.5 (94.0) |
| % Energy | 73.4 (7.2) b | 73.4 (6.5) b | 73.9 (6.6) b | 72.7 (6.9) b | 72.2 (6.6) | 72.6 (7.2) b | 74.5 (6.4) b |
| Fiber | 5.8 (3.0) | 6.1 (3.0) | 6.2 (3.1) a | 5.9 (3.0) | 6.0 (3.0) | 6.1 (3.1) | 6.1 (3.2) |
| Micronutrients | |||||||
| Vitamin A, R.E. | 478.7 (356.8) | 497.2 (338.3) | 502.4 (353.1) | 483.4 (341.6) | 485.4 (331.9) | 511.7 (367.1) | 51.89 (401.1) |
| Vitamin B1, mg | 0.93 (0.48) a | 0.95 (0.39) | 0.92 (0.40) a | 0.94 (0.41) a | 0.97 (0.42) | 0.98 (0.42) | 0.91 (0.42) |
| Vitamin B2, mg | 0.82 (0.43) a | 0.88 (0.43) | 0.88 (0.42) | 0.88 (0.42) | 0.91 (0.45) | 0.91 (0.45) | 0.83 (0.46) |
| Niacin, mg | 13.8 (6.4) | 13.9 (5.6) | 13.5 (5.6) | 13.6 (6.0) | 14.4 (5.8) | 14.4 (6.2) | 13.2 (5.7) |
| Folate, µg | 217.7 (122.3) | 226.1 (124.3) | 233.8 (127.1) | 223.7 (122.4) | 223.9 (120.7) | 231.4 (128.8) | 232.7 (153.2) |
| Vitamin B6, mg | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) |
| Vitamin C, mg | 105.2 (66.3) | 109.8 (63.1) | 120.7 (76.9) | 113.7 (74.8) | 114.3 (71.2) | 115.0 (76.0) | 120.8 (105.1) |
| Vitamin E, mg | 7.9 (4.3) | 8.1 (4.2) | 8.3 (4.5) | 8.1 (4.5) | 8.4 (5.0) | 8.4 (4.7) | 7.9 (4.7) |
| Minerals | |||||||
| Calcium, mg | 405.0 (243.9) a | 443.1 (264.5) | 463.4 (266.7) | 456.0 (255.2) | 475.1 (281.2) | 462.0 (275.8) | 444.6 (294.4) |
| Phosphorus, mg | 843.4 (349.6) a | 871.0 (353.0) | 861.3 (340.3) | 865.2 (344.4) | 903.3 (366.4) | 893.2 (367.6) | 842.9 (359.5) |
| Iron, mg | 9.9 (5.2) | 10.2 (5.0) | 10.3 (5.2) | 9.9 (4.8) | 10.3 (5.0) | 10.4 (5.4) | 10.0 (5.4) |
| Potassium, mg | 2157.1 (1050.3) | 227.5 (1046.9) | 2313.2 (1100.0) | 2251.5 (1084.9) | 2306.9 (1100.7) | 2326.4 (1128.2) | 2262.2 (1248.3) |
| Zinc, µg | 7.6 (3.4) | 7.9 (3.2) | 7.6 (3.4) | 7.6 (3.3) | 7.9 (3.1) | 8.1 (4.0) | 7.6 (3.1) |
| Nutrient | Non-Cancer I N = 19,926 | Odds Ratio a (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gastric Cancer | Colon Cancer | Breast Cancer | Cervical Cancer | Thyroid Cancer | Other Cancer | Multiple Cancers | ||
| N = 727 | N = 372 | N = 864 | N = 603 | N = 880 | N = 1405 | N = 136 | ||
| Total energy | Reference | 0.83 (0.69–1.02) | 0.82 (0.62–1.08) | 0.89 (0.76–1.05) | 0.90 (0.74–1.09) | 0.94 (0.80–1.11) | 0.98 (0.86–1.11) | 0.85 (0.56–1.30) |
| Carbohydrate (% of total energy) | 1.23 (0.95–1.61) | 1.50 (1.03–2.18) | 1.83 (1.40–2.39) | 0.87 (0.66–1.13) | 1.15 (0.92–1.43) | 1.08 (0.92–1.28) | 1.83 (0.93–3.60) | |
| Fat (% of total energy) | - b | - b | - b | - b | - b | - b | - b | |
| Protein | 0.76 (0.64–0.91) | 0.88 (0.69–1.11) | 0.79 (0.67–0.93) | 0.78 (0.65–0.95) | 1.03 (0.87–1.20) | 0.85 (0.76–0.96) | 0.72 (0.48–1.07) | |
| Vitamin A | 1.01 (0.81–1.26) | 1.13 (0.85–1.52) | 1.03 (0.86–1.23) | 0.96 (0.77–1.20) | 0.95 (0.79–1.14) | 1.14 (0.99–1.30) | 0.90 (0.58–1.39) | |
| Vitamin B1 | 0.79 (0.64–0.97) | 0.76 (0.64–0.99) | 0.75 (0.62–0.89) | 0.81 (0.66–1.01) | 0.97 (0.82–1.15) | 0.95 (0.84–1.08) | 0.66 (0.41–1.04) | |
| Vitamin B2 | 0.84 (0.64–1.10) | 0.77 (0.53–1.12) | 0.83 (0.68–1.02) | 0.89 (0.70–1.14) | 0.94 (0.76–1.15) | 1.02 (0.87–1.20) | 0.53 (0.29–0.97) | |
| Niacin, mg | 0.90 (0.75–1.08) | 1.01 (0.78–1.29) | 0.76 (0.65–0.90) | 0.87 (0.71–1.05) | 0.96 (0.82–1.13) | 0.96 (0.85–1.08) | 0.66 (0.44–1.01) | |
| Folate | 1.10 (0.79–1.54) | 0.90 (0.49–1.31) | 0.93 (0.70–1.23) | 0.99 (0.70–1.38) | 1.14 (0.85–1.51) | 1.25 (1.02–1.54) | 1.09 (0.58–2.04) | |
| Vitamin B6 | 1.01 (0.85–1.20) | 1.12 (0.88–1.42) | 0.94 (0.80–1.10) | 0.92 (0.77–1.12) | 1.15 (0.98–1.35) | 0.98 (0.87–1.10) | 0.84 (0.57–1.25) | |
| Vitamin C | 1.25 (1.05–1.49) | 1.28 (1.01–1.63) | 1.15 (0.98–1.34) | 1.17 (0.97–1.42) | 1.08 (0.92–1.26) | 1.19 (1.05–1.33) | 1.01 (0.68–1.51) | |
| Calcium | 0.79 (0.59–1.08) | 0.91 (0.60–1.37) | 0.79 (0.62–1.02) | 0.99 (0.73–1.35) | 1.27 (1.00–1.60) | 1.08 (0.90–1.30) | 0.84 (0.44–1.63) | |
| Phosphorus | 0.78 (0.65–0.94) | 0.83 (0.65–1.07) | 0.81 (0.68–0.96) | 0.76 (0.63–0.93) | 1.09 (0.92–1.29) | 0.85 (0.75–0.96) | 0.73 (0.49–1.11) | |
| Iron | 1.04 (0.87–1.25) | 1.05 (0.82–1.34) | 1.04 (0.88–1.24) | 0.99 (0.81–1.21) | 1.18 (1.00–1.40) | 1.03 (0.91–1.16) | 0.86 (0.57–1.29) | |
| Zinc | 0.79 (0.65–0.95) | 0.91 (0.70–1.17) | 0.93 (0.79–1.09) | 0.95 (0.78–1.14) | 1.08 (0.92–1.26) | 0.99 (0.88–1.11) | 0.67 (0.45–1.01) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Lee, J.; Kim, J. Imbalanced Nutrient Intake in Cancer Survivors from the Examination from the Nationwide Health Examination Center-Based Cohort. Nutrients 2018, 10, 212. https://doi.org/10.3390/nu10020212
Park B, Lee J, Kim J. Imbalanced Nutrient Intake in Cancer Survivors from the Examination from the Nationwide Health Examination Center-Based Cohort. Nutrients. 2018; 10(2):212. https://doi.org/10.3390/nu10020212
Chicago/Turabian StylePark, Boyoung, Jinhee Lee, and Jeongseon Kim. 2018. "Imbalanced Nutrient Intake in Cancer Survivors from the Examination from the Nationwide Health Examination Center-Based Cohort" Nutrients 10, no. 2: 212. https://doi.org/10.3390/nu10020212





