Differential Long-Chain Polyunsaturated Fatty Acids Status and Placental Transport in Adolescent Pregnancies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Processing
2.3. Fatty Acids Analysis
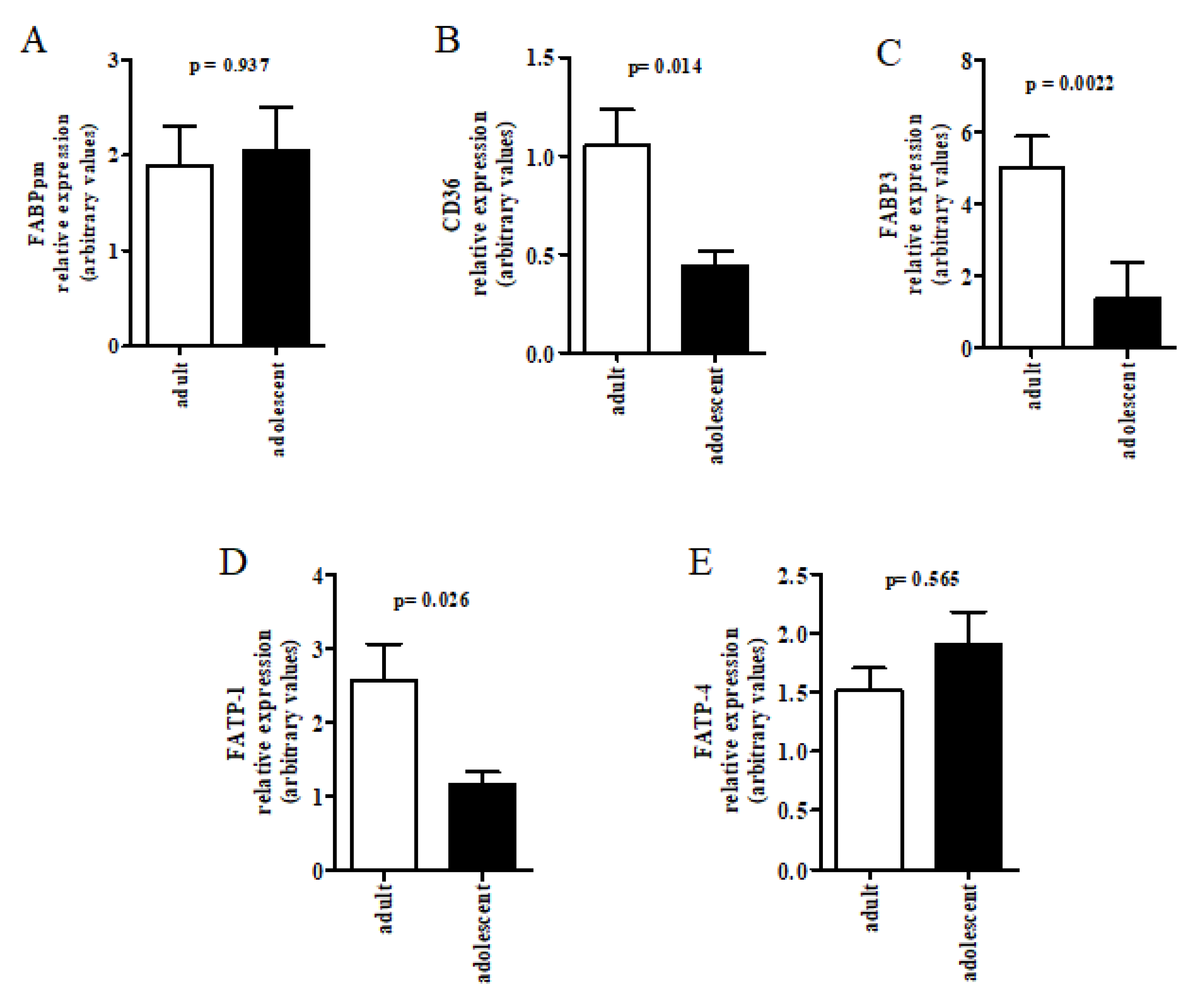
2.4. Placental Gene Expression Analysis
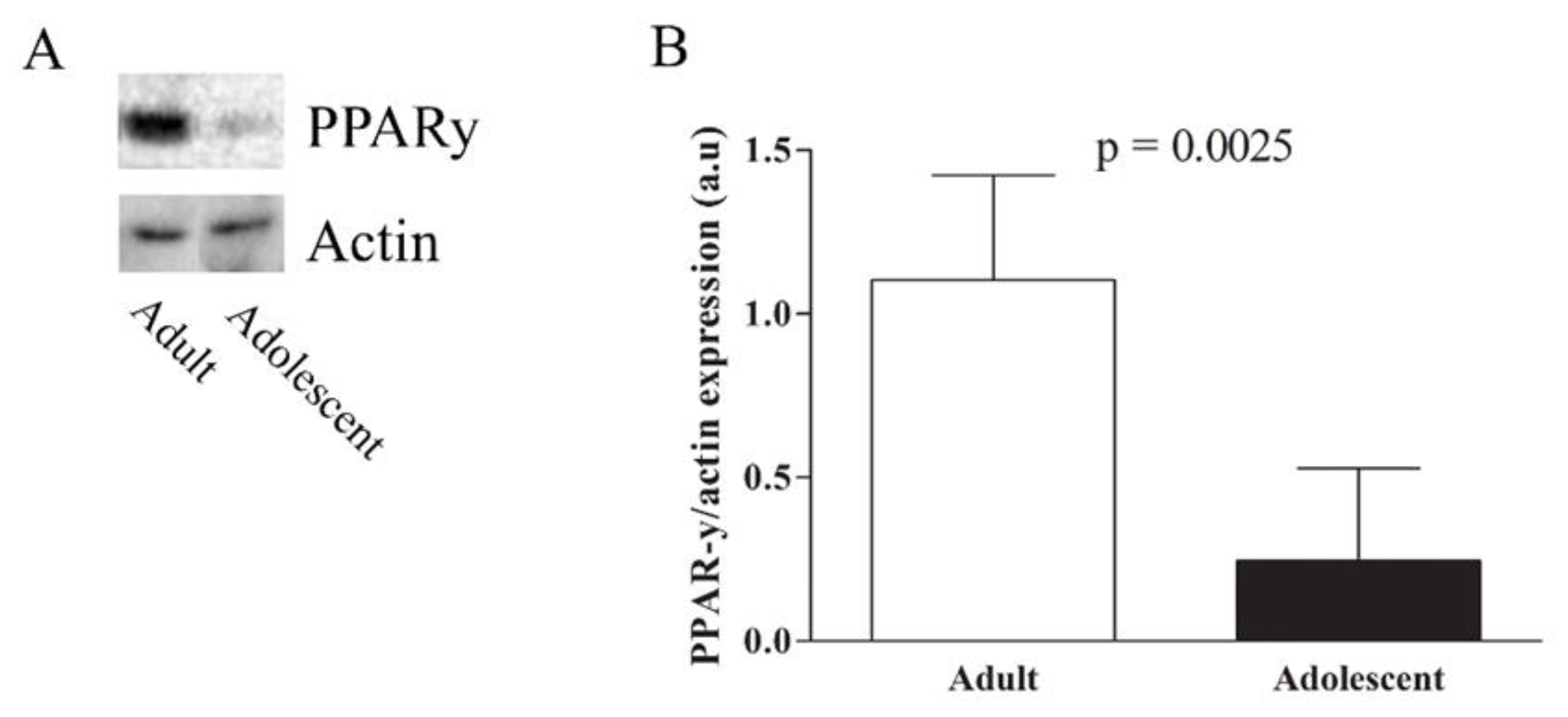
2.5. Western Blot
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Innis, S.M. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003, 143, S1–S8. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, A.L.; Waterman, I.J.; Wennergren, M.; Jansson, T.; Powell, T.L. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4607–4614. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.M.; Bush, P.G.; Veerkamp, J.H.; Dutta-Roy, A.K. Detection and cellular localization of plasma membrane-associated and cytoplasmic fatty acid-binding proteins in human placenta. Placenta 1998, 19, 409–415. [Google Scholar] [CrossRef]
- Koonen, D.P.; Glatz, J.F.; Bonen, A.; Luiken, J.J. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim. Biophys. Acta 2005, 1736, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.M.; Gordon, M.J.; Dutta-Roy, A.K. Preferential uptake of long chain polyunsaturated fatty acids by isolated human placental membranes. Mol. Cell. Biochem. 1996, 155, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hanebutt, F.L.; Demmelmair, H.; Schiess, B.; Larque, E.; Koletzko, B. Long-chain polyunsaturated fatty acid (LCPUFA) transfer across the placenta. Clin. Nutr. 2008, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.M.; Gordon, M.J.; Dutta-Roy, A.K. Placental membrane fatty acid-binding protein preferentially binds arachidonic and docosahexaenoic acids. Life Sci. 1998, 63, 235–240. [Google Scholar] [CrossRef]
- Larqué, E.; Krauss-Etschmann, S.; Campoy, C.; Hartl, D.; Linde, J.; Klingler, M.; Demmelmair, H.; Caño, A.; Gil, A.; Bondy, A.B.; et al. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am. J. Clin. Nutr. 2006, 84, 853–861. [Google Scholar] [PubMed]
- Storch, J.; McDermott, L. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 2009, 50, S126–S131. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, A.; Demmelmair, H.; Parrilla, J.J.; Koletzko, B.; Larqué, E. Mechanisms involved in the selective transfer of long chain polyunsaturated fatty acids to the fetus. Front. Genet. 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Kagawa, Y.; Sharifi, K.; Ebrahimi, M.; Miyazaki, H.; Yasumoto, Y.; Kawamura, S.; Yamamoto, Y.; Sakaguti, S.; Sawada, T.; et al. Fatty acid binding protein 3 is involved in n-3 and n-6 PUFA transport in mouse trophoblasts –3. J. Nutr. 2014, 144, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Cook, T.J.; Knipp, G.T. Effect of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcomes. J. Pharm. Sci. 2007, 96, 2582–2606. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Fatty acid-activated nuclear transcription factors and their roles in human placenta. Eur. J. Lipid Sci. Technol. 2006, 108, 70–83. [Google Scholar] [CrossRef]
- Ministério da Saúde—MS—Brasil. Pesquisa Nacional da Demografia e Saúde da Criança e da Mulher—PNDS 2006; Brasília-DF. 2008. Available online: http://bvsms.saude.gov.br/bvs/pnds/index.php (accessed on 4 December 2017).
- Wallace, J.M.; Luther, J.S.; Milne, J.S.; Aitken, R.P.; Redmer, R.A.; Reynolds, L.P.; Hay, W.W. Nutritional modulation of adolescent pregnancy outcome—A review. Placenta 2006, 27, S61–S68. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.K.; Wen, S.W.; Fleming, N.; Demissie, K.; Rhoads, G.G.; Walker, M. Teenage pregnancy and adverse birth outcomes: A large population-based retrospective cohort study. Int. J. Epidemiol. 2007, 36, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.E.; Greenwood, S.L.; Sibley, C.P.; Baker, P.N.; Jones, R.L. Effect of young maternal age and skeletal growth on placental growth and development. Placenta 2011, 32, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.E.; Greenwood, S.L.; Sibley, C.P.; Baker, P.N.; Challis, J.R.; Jones, R.L. Effect of maternal age and growth on placental nutrient transport: Potential mechanisms for teenagers’ predisposition to small-for-gestational-age birth? Am. J. Physiol. Endocrinol. Metab. 2012, 302, E233–E242. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.R.C.; Santana, M.G.; Santos, F.S.; Conceição, F.; Sardinha, F.L.C.; Veiga, G.V.; Tavares do Carmo, M.G. Composition of fatty acids in the maternal and umbilical cord plasma of adolescent and adult mothers: Relationship with anthropometric parameters of newborn. Lipids Health Dis. 2012, 11, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ghebremeskel, K.; Min, Y.; Crawford, M.A.; Nam, J.H.; Kim, A.; Koo, J.N.; Suzuki, H. Blood fatty acid composition of pregnant and nonpregnant Korean women: Red cells may act as a reservoir of arachidonic acid and docosahexaenoic acid for utilization by the developing fetus. Lipids 2000, 35, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ruyle, M.; Connor, W.E.; Anderson, G.J.; Lowensohn, R.I. Placental transfer of essential fatty acids in humans: Venous-arterial difference for docosahexaenoic acid in fetal umbilical erythrocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 7902–7906. [Google Scholar] [CrossRef] [PubMed]
- National Health Council- Brazil: Resolution No. 196/96—Guidelines and Rules for Research Involving Human. Bioethics 1996. Available online: http://www.ufrgs.br/bioetica/res19696.htm (accessed on 4 December 2017).
- Broekhuyse, R.M. Long-term storage of erythrocytes for quantitative analyses of lipids. Clin. Chim. Acta 1974, 52, 53–58. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipid in a one etep reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- De Velasco, P.C.; Chicaybam, G.; Ramos-Filho, D.M.; dos Santos, R.M.A.R.; Mairink, C.; Sardinha, F.L.C.; El-Bacha, T.; Galina, A.; Tavares-do-Carmo, M.G. Maternal intake of trans-unsaturated or interesterified fatty acids during pregnancy and lactation modifies mitochondrial bioenergetics in the liver of adult offspring in mice. Br. J. Nutr. 2017, 118, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent); CSHL Press: Cold Spring Harbor, NY, USA, 2010. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle—Dye binding. Anal. Biochem. 1976, 7, 48–54. [Google Scholar]
- Meneses, F.; Ney, J.G.; Torres, A.G.; Trugo, N.M.F. Erythrocyte membrane and plasma non-esterified n-3 and n-6 polyunsaturated fatty acids of pregnant and nonpregnant Brazilian adolescents. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Trugo, N.M.F. Evidence of inadequate docosahexaenoic acid status in Brazilian pregnant and lactating women. Rev. Saúde Pública 2009, 43, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.S.; Santos, F.S.; Mucci, D.B.; Souza, T.V.; Sardinha, F.L.C.; Chaves, C.R.M.M.; Tavares-do-Carmo, M.G. Trans fatty acids in colostrum, mature milk and diet of lactating adolescents. Lipids 2016, 51, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Effect of placental function on fatty acid requirements during pregnancy. Eur. J. Clin. Nutr. 2004, 58, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.; Mosandl, A.; Bursen, A.; Marschalek, R.; Sewell, A.C.; Bohles, H. Multidimensional gas chromatography-mass spectrometry for tracer studies of fatty acid metabolism via stable isotopes in cultured human trophoblast cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 791, 235–244. [Google Scholar] [CrossRef]
- Haggarty, P.; Pagec, K.; Abramovich, D.R.; Ashton, J.; Browr, D. Long-chain polyunsaturated fatty acid transport across the perfused human placenta. Placenta 1997, 18, 635–642. [Google Scholar] [CrossRef]
- Lattka, E.; Koletzko, B.; Zeilinger, S.; Hibbeln, J.R.; Klopp, N.; Ring, S.M.; Steer, C.D. Umbilical cord PUFA are determined by maternal and child fatty acid desaturase (FADS) genetic variants in the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 2013, 109, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.T.; Gordon, M.J.; Campbell, F.M.; Dutta-Roy, A.K. Differential distribution and metabolism of arachidonic acid and docosahexaenoic acid by human placental choriocarcinoma (BeWo) cells. Mol. Cell. Biochem. 1998, 185, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bitsanis, D.; Crawford, M.A.; Moodley, T.; Holmsen, H.; Ghebremeskel, K.; Djahanbakhch, O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J. Nutr. 2005, 135, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.R.; Sloboda, D.M.; Alfaidy, N.; Lye, S.J.; Gibb, W.; Patel, F.A.; Whittle, W.L.; Newnham, J.P. Prostaglandins and mechanisms of preterm birth. Reproduction 2002, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, S.; Rousseau, E.; Berthiaume, M.; Pasquier, J.C. Lipoxygenase and cyclooxygenase inhibitors reveal a complementary role of arachidonic acid derivatives in pregnant human myometrium. Am. J. Obstet. Gynecol. 2010, 203, 266.e1–266.e7. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Liu, G.; Kavuma, E.; Hewson, S.A.; McKay, D.; Hannah, M.E. Preterm labour and birth: A survey of clinical practice regarding use of tocolytics, antenatal corticosteroids, and progesterone. J. Obstet. Gynaecol. Can. 2007, 29, 117–130. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.; González, R.S.; Maldonado, J.; López-Alarcón, M.; Bernabe-García, M. The effect of gestational age on expression of genes involved in uptake, trafficking and synthesis of fatty acids in the rat placenta. Gene 2016, 591, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Frank, B.H. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [PubMed]
- Spitsberg, V.L.; Matitashvili, E.; Gorewit, R.C. Association and coexpression of fatty-acid-binding protein and glycoprotein CD36 in the bovine mammary gland. Eur. J. Biochem. 1995, 230, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhoven, F.A.; Willemsen, P.H.; Van der Vusse, G.J.; Glatz, J.F. Co-expression in rat heart and skeletal muscle of four genes coding for proteins implicated in long-chain fatty acid uptake. Int. J. Biochem. Cell Biol. 1999, 31, 489–498. [Google Scholar] [CrossRef]
- Hanhoff, T.; Lücke, C.; Spener, F. Insights into binding of fatty acids by fatty acid binding proteins. Mol. Cell. Biochem. 2002, 239, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.M.; Clohessy, A.M.; Gordon, K.R.; Page, A.K. Uptake of long chain fatty acids by human placental choriocarcinoma (BeWo) cells: Role of plasma membrane fatty acid binding protein. J. Lipid Res. 1997, 38, 2558–2568. [Google Scholar] [PubMed]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Schaiff, W.T.; Knapp, F.F.; Barak, Y.; Biron-Shental, T.; Nelson, D.M.; Sadovsky, Y. Ligand-activated peroxisome proliferator activated receptor γ alters placental morphology and placental fatty acid uptake in mice. Endocrinology 2007, 148, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Schaiff, W.T.; Bildirici, I.; Cheong, M.; Chern, P.L.; Nelson, D.M.; Sadovsky, Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J. Clin. Endocrinol. Metab. 2005, 90, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fairall, L.; Amin, K.; Inaba, Y.; Szanto, A.; Balint, B.L.; Nagy, L.; Yamamoto, K.; Schwabe, J.W.R. Structural basis for the activation of PPARγ by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008, 15, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Zand, H.; Rhimipour, A.; Bakhshayesh, M.; Shafiee, M.; Nour Mohammadi, I.; Salimi, S. Involvement of PPAR-c and p53 in DHA-induced apoptosis in Reh cells. Mol. Cell. Biochem. 2007, 304, 71–77. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| FAT/CD36 | GGAAAGTCACTGCGACATGA | CCTTGGATGGAAGAACGAATC |
| FABPpm | GGAAGGAAATAGCAACAGTGG | TCCTACACGCTCACCATATAAGC |
| FATP1 | AGGTGGTTCAGTACATCGGG | AGAACTCCCCGATTTGGC |
| FATP4 | ATACCCACTGAACCTTTGGC | AAGGTCTCTGTGGTGGCCAA |
| FABP3 | TTTTGCTACCAGGCAGGTG | TCATCTGCTGTTGTCTCATCG |
| GAPDH | GAAGGTGAAGGTCGGAGTCAA | GGAAGATGGTGATGGGATTTC |
| Variables | Adult (n = 15) | Adolescent (n = 15) |
|---|---|---|
| Maternal age (year) (mean ± SD (range)) | 25.6 ± 5.5 (20–35) | 16.9 ± 1.1 (15–19) |
| Per capita household income (n (%)) | ||
| <1 minimum wage | 10 (66.7) | 10 (66.7) |
| ≥1 minimum wage | 3 (20) | 2 (13.3) |
| Unable to inform | 2 (13.3) | 3 (20) |
| Marital status (n (%)) | ||
| Single | 5 (33.3) | 12 (80) |
| Married or co-habiting | 10 (66.7) | 3 (20) |
| Education (n (%)) | ||
| <8 years | 4 (26.6) | 9 (60) |
| ≥8 years | 11 (73.3) | 6 (40) |
| Employment during pregnancy (n (%)) | ||
| Yes | 12 (80) | 1 (6.7) |
| No | 3 (20) | 14 (93.3) |
| Parity (n (%)) | ||
| No delivery | 8 (53.3) | 13 (86.7) |
| ≥1 delivery | 7 (46.7) | 2 (13.3) |
| Prenatal follow-up visits (n (%)) | ||
| <6 | 1 (7) | 2 (13.3) |
| ≥6 | 14 (93) | 13 (86.7) |
| Variables | Adult | Adolescent |
|---|---|---|
| Maternal Characteristics | ||
| Pre-pregnancy nutritional status (n (%)) a | ||
| Underweight | 0 | 0 |
| Normal weight | 10 (66.7) | 11 (78.6) |
| Overweight | 5 (33.3) | 4 (21.4) |
| Gestational weight gain (n (%)) a | ||
| Insufficient | 1 (6.7) | 4 (28.0) |
| Recommended | 8 (53.3) | 6 (36.0) |
| Excessive | 6 (40.0) | 5 (36.0) |
| Delivery mode (n (%)) | ||
| Vaginal | 11 (73.3) | 11 (73.3) |
| Cesarean section | 4 (26.7) | 4 (26.7) |
| Neonatal characteristics | ||
| Newborn sex (n (%)) | ||
| Females | 7 (46.7) | 10 (66.7) |
| Males | 8 (53.3) | 5 (33.3) |
| Birth weight (g) (means ± SD) | 3347 ± 350 | 3395 ± 346 |
| Length at birth (cm) (means ± SD) | 47.4 ± 5.9 | 48.6 ± 1.5 |
| Head circumference (cm) (means ± SD) | 34.6 ± 3.7 | 34.3 ± 1.6 |
| Placental weight (g) (means ± SD) | 661 ± 130 | 610 ± 103 |
| Gestational age at delivery (weeks) | 39.9 ± 0.8 | 38.9 ± 0.7 * |
| Birth weight classification (n (%)) b | ||
| SGA | 0 | 0 |
| AGA | 15 (100) | 15 (100) |
| LGA | 0 | 0 |
| Placenta | |||
|---|---|---|---|
| Fatty acid (mg/100mg fatty acids) | Adult | Adolescent | p |
| Saturated (SFA) | |||
| 16:0 | 25.0 ± 3.5 | 23.0 ± 1.1 | 0.06 |
| 18:0 | 13.6 ± 1.2 | 13.3 ± 1.0 | 0.38 |
| Monounsaturated (MUFA) | |||
| 18:1 n-9 cis | 8.5 ± 1.5 | 11.0 ± 1.6 | 0.01 |
| 18:1 trans * | 0.95 ± 0.8 | 1.1 ± 0.6 | 0.39 |
| Essential polyunsaturated (EFA) | |||
| 18:2 n-6 (LA) | 8.4 ± 1.5 | 9.6 ± 1.0 | 0.01 |
| 18:3 n-3 (ALA) | 0.80 ± 0.4 | 0.92 ± 0.3 | 0.41 |
| Long-chain polyunsaturated (LCPUFA) | |||
| 20:4 n-6 (AA) | 14.6 ± 4.5 | 17.2 ± 1.6 | 0.04 |
| 22:4 n-6 | 0.98 ± 0.2 | 0.90 ± 0.3 | 0.94 |
| 20:5 n-3 (EPA) | 0.44 ± 0.2 | 0.29 ± 0.1 | 0.35 |
| 22:6 n-3 (DHA) | 2.4 ± 0.6 | 1.3 ± 0.9 | 0.001 |
| 22:5 n-3 (DPA) | 0.87 ± 0.5 | 0.56 ± 0.4 | 0.23 |
| Total SFA a | 44.5 ± 7.0 | 42.1 ± 2.4 | 0.21 |
| Total MUFA b | 18.8 ± 4.0 | 25.1 ± 4.4 | 0.04 |
| Total EFA c | 9.2 ± 1.7 | 10.6 ± 1.1 | 0.01 |
| Total LCPUFA d | 22.0 ± 6.2 | 22.8 ± 5.4 | 0.73 |
| Total PUFA n-6 e | 26.7 ± 1.9 | 31.3 ± 5.4 | 0.04 |
| Total PUFA n-3 f | 5.8 ± 0.4 | 4.3 ± 1.1 | 0.01 |
| Total n-6/n-3 ratio | 5.0 ± 1.3 | 7.7 ± 2.4 | 0.0007 |
| AA/LA n-6 ratio | 1.75 ± 0.6 | 1.78 ± 0.1 | 0.88 |
| DHA/ALA n-3 ratio | 4.8 ± 2.8 | 1.5 ± 1.0 | 0.01 |
| Maternal Erythrocytes | Umbilical Cord Erythrocytes | |||
|---|---|---|---|---|
| Fatty Acids (mg/100 mg) | Adult | Adolescent | Adult | Adolescent |
| Saturated (SFA) | ||||
| 14:0 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.2 ** |
| 16:0 | 23.5 ±1.4 | 23.6 ± 1.2 | 23.9 ± 0.9 | 25.8 ± 2.4 *,# |
| 18:0 | 17.4 ± 0.8 | 17.7 ± 1.2 | 18.5 ± 0.5 *** | 19.4 ± 1.8 ** |
| 22:0 | 0.8 ± 0.13 | 0.7 ± 0.20 | 0.7 ± 0.1 | 0.8 ± 0.45 |
| Monounsaturated (MUFA) | ||||
| 18:1 n-9 cis | 12.5 ± 1.3 | 11.9 ± 1.0 | 10.9 ± 1.2 *** | 10.4 ± 1.7 *** |
| 18:1 n-7 cis | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.4 ± 0.2 | 1.5 ± 0.3 |
| Essential polyunsaturated (EFA) | ||||
| 18:2 n-6 (LA) | 11.0 ± 1.8 | 12.0 ± 1.0 | 4.2 ± 0.6 *** | 4.3 ± 0.7 *** |
| 18:3 n-3 (ALA) | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Long-chain polyunsaturated (LCPUFA) | ||||
| 20:4 n-6 (AA) | 15.8 ± 1.6 | 14.7 ± 1.4 | 20.0 ± 1.6 *** | 17.6 ± 2.5 ***,# |
| 22:4 n-6 | 3.8 ± 0.6 | 4.5 ± 0.7 # | 4.3 ± 0.4 | 4.1 ± 1.0 * |
| 20:5 n-3 (EPA) | 0.7 ± 0.3 | 0.5 ± 0.3 | 0.8 ± 0.1 | 0.8 ± 0.2 * |
| 22:6 n-3 (DHA) | 5.5 ± 1.3 | 4.5 ± 0.5 # | 6.1 ± 0.9 | 5.5 ± 1.0 ** |
| 22:5 n-3 (DPA) | 1.9 ± 0.5 | 1.8 ± 0.3 | 0.5 ± 0.2 *** | 0.6 ± 0.2 *** |
| Total SFA a | 41.8 ± 2.7 | 42.3 ± 1.9 | 43.7 ± 1.0* | 47.5 ± 4.4 ** |
| Total MUFA b | 13.8 ±1.4 | 13.1 ± 0.9 | 12.3 ± 1.3 | 12.0 ± 1.8 |
| Total EFA c | 11.5 ± 1.8 | 12.5 ± 1.0 | 4.5 ± 0.6 *** | 4.6 ± 0.6 *** |
| Total LCPUFA d | 30.3 ± 3.2 | 28.0 ± 2.3 # | 34.9 ± 1.8 *** | 31.5 ± 3.5 ***,## |
| Total PUFA n-6 e | 33.5 ± 2.4 | 33.8 ± 2.3 | 32.0 ± 1.8 | 30.0 ± 1.4 * |
| Total PUFA n-3 f | 8.5 ±1.2 | 7.2 ± 0.6 ## | 7.7 ± 1.1 | 7.2 ± 1.0 |
| Total n-6/n-3 ratio | 4.1 ± 0.8 | 4.7 ± 0.5 # | 4.3 ± 0.8 | 4.1 ± 0.7 * |
| AA/LA n-6 ratio | 1.5 ± 0.7 | 1.3 ± 0.2 | 4.8 ± 0.5 | 4.2 ± 0.7 |
| DHA/ALA n-3 ratio | 18.4 ± 1.6 | 11.4 ± 1.5 # | 30.5 ± 2.7 *** | 18.3 ± 1.7 ***,# |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, F.C.P.d.; Mucci, D.D.B.; Assumpção, R.P.; Marcondes, H.; Sardinha, F.L.d.C.; Silva, S.V.; Citelli, M.; Tavares do Carmo, M.D.G. Differential Long-Chain Polyunsaturated Fatty Acids Status and Placental Transport in Adolescent Pregnancies. Nutrients 2018, 10, 220. https://doi.org/10.3390/nu10020220
Fonseca FCPd, Mucci DDB, Assumpção RP, Marcondes H, Sardinha FLdC, Silva SV, Citelli M, Tavares do Carmo MDG. Differential Long-Chain Polyunsaturated Fatty Acids Status and Placental Transport in Adolescent Pregnancies. Nutrients. 2018; 10(2):220. https://doi.org/10.3390/nu10020220
Chicago/Turabian StyleFonseca, Fernanda Carrilho Pinto da, Daniela De Barros Mucci, Renata Pereira Assumpção, Henrique Marcondes, Fátima Lúcia de Carvalho Sardinha, Simone Vargas Silva, Marta Citelli, and Maria Das Graças Tavares do Carmo. 2018. "Differential Long-Chain Polyunsaturated Fatty Acids Status and Placental Transport in Adolescent Pregnancies" Nutrients 10, no. 2: 220. https://doi.org/10.3390/nu10020220





