Diurnal Variation of Sweet Taste Recognition Thresholds Is Absent in Overweight and Obese Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Experimental Protocol
2.2. Taste Recognition Threshold Measurement
2.3. Assays
2.4. Data Analysis
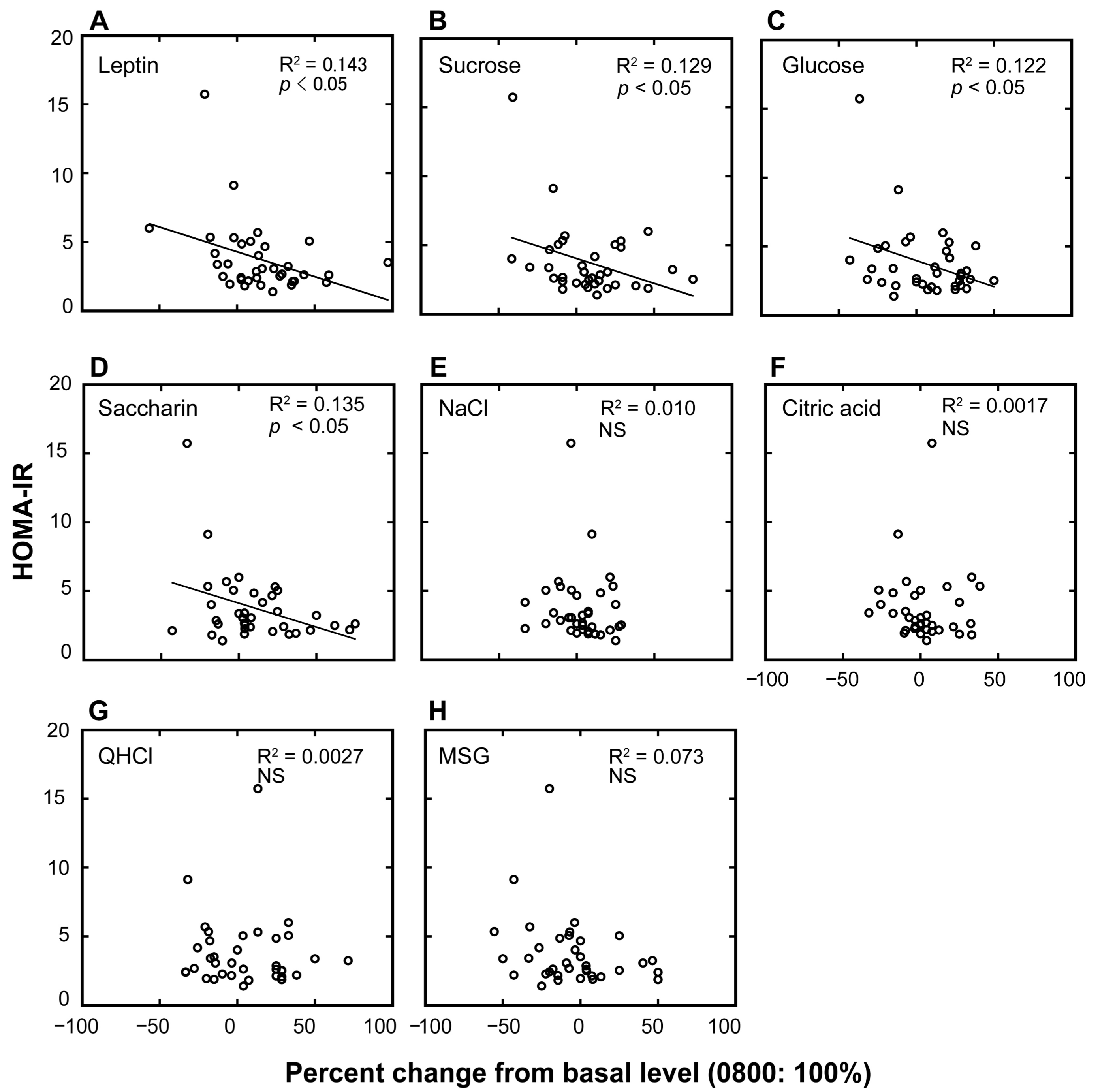
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Sugimoto, K.; Nakashima, K.; Miura, H.; Ninomiya, Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, N.; Ohta, R.; Kusakabe, Y.; Miura, H.; Hino, A.; Koyano, K.; Nakashima, K.; Ninomiya, Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 2004, 145, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Sanematsu, K.; Ohta, R.; Shirosaki, S.; Koyano, K.; Nonaka, K.; Shigemura, N.; Ninomiya, Y. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes 2008, 57, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Niki, M.; Jyotaki, M.; Yoshida, R.; Yasumatsu, K.; Shigemura, N.; DiPatrizio, N.V.; Piomelli, D.; Ninomiya, Y. Modulation of sweet taste sensitivities by endogenous leptin and endocannabinoids in mice. J. Physiol. 2015, 593, 2527–2545. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Noguchi, K.; Shigemura, N.; Jyotaki, M.; Takahashi, I.; Margolskee, R.F.; Ninomiya, Y. Leptin Suppresses Mouse Taste Cell Responses to Sweet Compounds. Diabetes 2015, 64, 3751–3762. [Google Scholar] [CrossRef] [PubMed]
- Belfort-DeAguiar, R.; Seo, D.; Lacadie, C.; Naik, S.; Schmidt, C.; Lam, W.; Hwang, J.; Constable, T.; Sinha, R.; Sherwin, R.S. Obese Humans Have Disordered Brain Responses to Food Images During Physiological Hyperglycemia. Am. J. Endocrinol. Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, S.; Bernal Fernández, G.; Martínez Salazar, M.F.; Monroy Noyola, A.; Toledano Jaimes, C.; Meneses Acosta, A.; Gonzalez Maya, L.; Aveleyra Ojeda, E.; Terrazas Meraz, M.A.; Boll, M.-C.; et al. Serum leptin is associated with metabolic syndrome in obese Mexican subjects. J. Clin. Lab. Anal. 2015, 29, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Kaiser, K.A.; Franks, S.F.; Deere, C.; Caffrey, J.L. Influence of BMI and gender on postprandial hormone responses. Obesity 2007, 15, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Ducroc, R.; Guilmeau, S.; Akasbi, K.; Devaud, H.; Buyse, M.; Bado, A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes 2005, 54, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Seufert, J.; Kieffer, T.J.; Leech, C.A.; Holz, G.G.; Moritz, W.; Ricordi, C.; Habener, J.F. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: Implications for the development of adipogenic diabetes mellitus. J. Clin. Endocrinol. Metab. 1999, 84, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Marchetti, P.; Maffei, M.; Del Guerra, S.; Benzi, L.; Marselli, L.; Bertacca, A.; Navalesi, R. Effects of acute or prolonged exposure to human leptin on isolated human islet function. Biochem. Biophys. Res. Commun. 1999, 256, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Hrebicek, J.; Janout, V.; Malincikova, J.; Horakova, D.; Cizek, L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J. Clin. Endocrinol. Metab. 2002, 87, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Fukushima, M.; Sakai, M.; Kataoka, K.; Miwa, K.; Nagata, I.; Doi, K.; Tokuyama, K.; Nakai, Y. Insulin-sensitive and insulin-resistant variants in nonobese Japanese type 2 diabetic patients. The role of triglycerides in insulin resistance. Diabetes Care 1999, 22, 2100–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.K.; Sukumaran, S.K.; Kotha, R.; Gilbertson, T.A.; Margolskee, R.F. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5431–5436. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.K.; Yee, K.K.; Iwata, S.; Kotha, R.; Quezada-Calvillo, R.; Nichols, B.L.; Mohan, S.; Pinto, B.M.; Shigemura, N.; Ninomiya, Y.; et al. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc. Natl. Acad. Sci. USA 2016, 113, 6035–6040. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Cummings, D.E.; Newby, P.D.; Breen, P.A.; Frayo, R.S.; Matthys, C.C.; Callahan, H.S.; Purnell, J.Q. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J. Clin. Endocrinol. Metab. 2003, 88, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef] [PubMed]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.H.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef] [PubMed]
- Young, R.L.; Chia, B.; Isaacs, N.J.; Ma, J.; Khoo, J.; Wu, T.; Horowitz, M.; Rayner, C.K. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 2013, 62, 3532–3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.L.; Sutherland, K.; Pezos, N.; Brierley, S.M.; Horowitz, M.; Rayner, C.K.; Blackshaw, L.A. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 2009, 58, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lostao, M.P.; Urdaneta, E.; Martínez-Ansó, E.; Barber, A.; Martínez, J.A. Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett. 1998, 423, 302–306. [Google Scholar] [CrossRef]
- Iñigo, C.; Patel, N.; Kellett, G.L.; Barber, A.; Lostao, M.P. Luminal leptin inhibits intestinal sugar absorption in vivo. Acta Physiol. 2007, 190, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jyotaki, M.; Sanematsu, K.; Shigemura, N.; Yoshida, R.; Ninomiya, Y. Leptin suppresses sweet taste responses of enteroendocrine STC-1 cells. Neuroscience 2016, 332, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Wang, Z.L.; Wang, R.M.; Hurley, J.D.; Smith, D.M.; Ghatei, M.A.; Withers, D.J.; Gardiner, J.V.; Bailey, C.J.; Bloom, S.R. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Investig. 1997, 100, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Poitout, V.; Rouault, C.; Guerre-Millo, M.; Briaud, I.; Reach, G. Inhibition of insulin secretion by leptin in normal rodent islets of Langerhans. Endocrinology 1998, 139, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, V.; Liu, Y.L.; Cawthorne, M.A.; Morton, N.M.; Davenport, M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 1997, 46, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, Y.; Okuya, S.; Ishihara, H.; Asano, T.; Yada, T.; Oka, Y. Direct stimulation of basal insulin secretion by physiological concentrations of leptin in pancreatic beta cells. Endocrinology 1997, 138, 4513–4516. [Google Scholar] [CrossRef] [PubMed]
- Echwald, S.M.; Clausen, J.O.; Hansen, T.; Urhammer, S.A.; Hansen, L.; Dinesen, B.; Borch-Johnsen, K. Analysis of the relationship between fasting serum leptin levels and estimates of beta-cell function and insulin sensitivity in a population sample of 380 healthy young Caucasians. Eur. J. Endocrinol. 1999, 140, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Carantoni, M.; McLaughlin, T.; Reaven, G.M. Plasma insulin concentration is more tightly linked to plasma leptin concentration than is the body mass index. Metabolism 2000, 49, 544–547. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Lollmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Leptin at 14 y of age: an ongoing story. Am. J. Clin. Nutr. 2009, 89, 973S–979S. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Obesity: Causes and control of excess body fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Chamberland, J.P.; Diakopoulos, K.N.; Fiorenza, C.G.; Ziemke, F.; Schneider, B.; Mantzoros, C.S. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care 2011, 34, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.-S.; Matarese, G.; Brennan, A.M.; Chamberland, J.P.; Liu, X.; Fiorenza, C.G.; Mylvaganam, G.H.; Abanni, L.; Carbone, F.; Williams, C.J.; et al. Efficacy of metreleptin in obese patients with type 2 diabetes: Cellular and molecular pathways underlying leptin tolerance. Diabetes 2011, 60, 1647–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, H.; Hanada, R.; Hanada, T.; Aki, D.; Mashima, R.; Nishinakamura, H.; Torisu, T.; Chien, K.R.; Yasukawa, H.; Yoshimura, A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004, 10, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H.; Flier, J.S.; Bjørbaek, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004, 145, 4880–4889. [Google Scholar] [CrossRef] [PubMed]
- Kievit, P.; Howard, J.K.; Badman, M.K.; Balthasar, N.; Coppari, R.; Mori, H.; Lee, C.E.; Elmquist, J.K.; Yoshimura, A.; Flier, J.S. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006, 4, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Könner, A.C.; Brüning, J.C. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol. Metab. 2011, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Könner, A.C.; Brüning, J.C. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012, 16, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.-Y.; Hamnvik, O.-P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef] [PubMed]
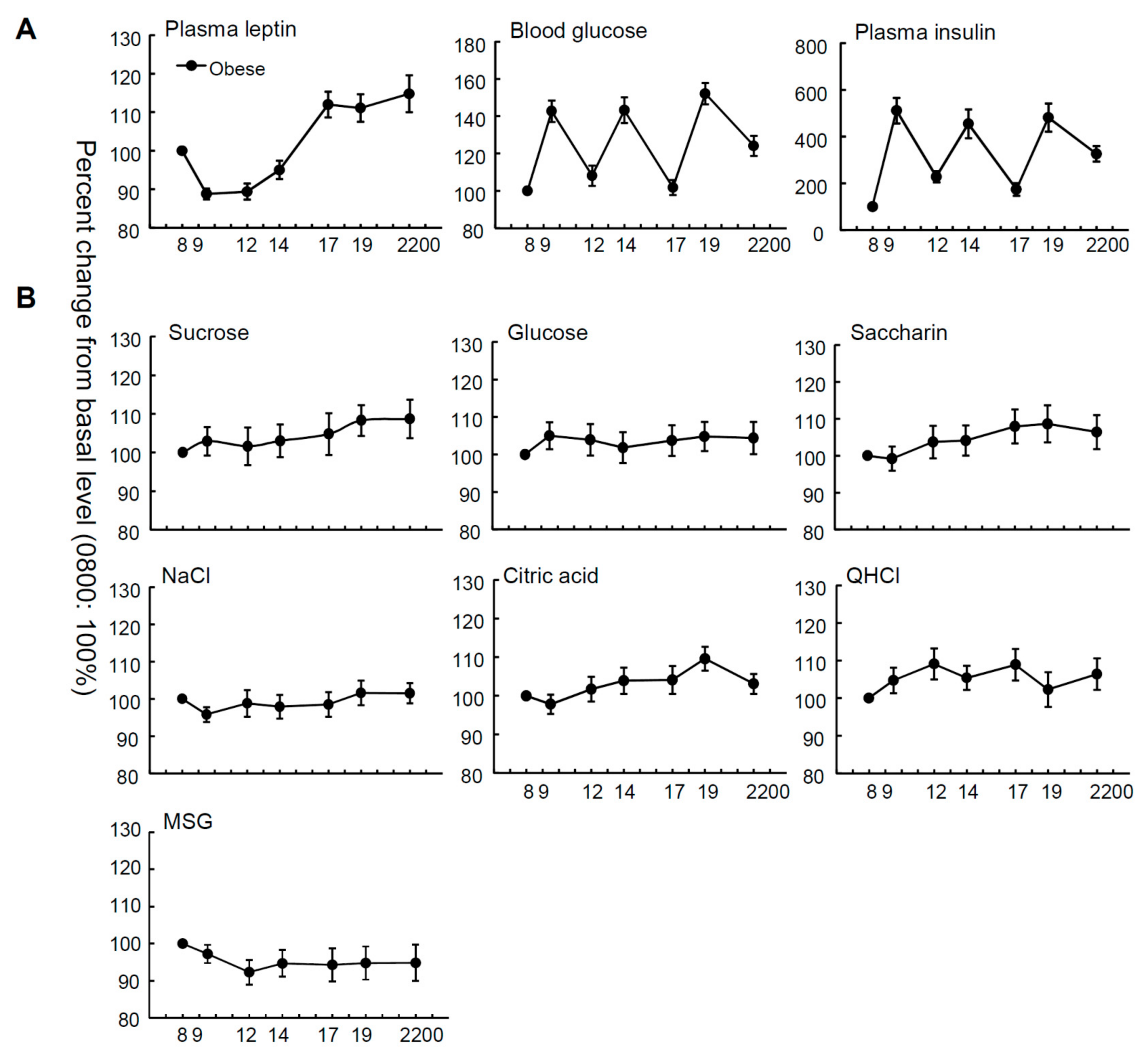
| Male (n = 17) | Female (n = 19) | t-Test | All Subjects (n = 36) | One-Way Repeated Measures ANOVA | ||
|---|---|---|---|---|---|---|
| p | F | p | ||||
| Leptin (ng/mL) | 12.3 ± 1.6 | 26.8 ± 4.4 | <0.01 | 20.2 ± 2.7 | (6, 245) = 16.18 | <0.001 |
| Blood glucose (mg/dL) | 97.7 ± 5.9 | 105.4 ± 8.4 | NS | 102.3 ± 5.2 | (6, 245) = 18.81 | <0.001 |
| Insulin (μIU/mL) | 13.6 ± 1.7 | 14.5 ± 1.9 | NS | 14.1 ± 1.2 | (6, 245) = 15.43 | <0.001 |
| HOMA-IR | 3.15 ± 0.46 | 4.18 ± 0.73 | NS | 3.69 ± 0.45 | ||
| QUICKI | 0.32 ± 0.005 | 0.32 ± 0.0047 | NS | 0.32 ± 0.0036 | ||
| BMI (kg/m2) | 30.8 ± 1.1 | 34.7 ± 1.9 | NS | 32.9 ± 1.1 | ||
| Taste Recognition Thresholds (mM) | ||||||
| Sucrose | 30.7± 3.3 | 23.4 ± 3.6 | NS | 26.8 ± 2.5 | (6, 243) = 0.66 | NS |
| Glucose | 131.4 ± 10.9 | 96.8 ± 17.1 | NS | 113.1 ± 10.6 | (6, 244) = 0.29 | NS |
| Saccharin | 0.12 ± 0.015 | 0.11 ± 0.020 | NS | 0.11 ± 0.011 | (6, 237) = 0.99 | NS |
| NaCl | 31.9 ± 3.8 | 27.2 ± 4.8 | NS | 29.4 ± 3.0 | (6, 237) = 0.66 | NS |
| citric acid | 0.56 ± 0.05 | 0.53 ± 0.08 | NS | 0.55 ± 0.05 | (6, 245) = 2.18 | NS |
| QHCl | 0.015 ± 0.003 | 0.015 ± 0.002 | NS | 0.015 ± 0.002 | (6, 245) = 0.99 | NS |
| MSG | 13.6 ± 2.8 | 9.9 ± 2.2 | NS | 11.5 ± 1.8 | (6, 231) = 0.53 | NS |
| HOMA-IR | |||
|---|---|---|---|
| β | Adjusted R2 | p | |
| a. plasma leptin | −0.379 | 0.12 | <0.05 |
| b. sucrose | −0.360 | 0.10 | <0.05 |
| c. glucose | −0.350 | 0.10 | <0.05 |
| d. saccharin | −0.367 | 0.11 | <0.05 |
| e. NaCl | −0.101 | −0.02 | NS |
| f. citric acid | −0.041 | −0.03 | NS |
| g. QHCl | −0.005 | −0.03 | NS |
| h. MSG | −0.269 | 0.05 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanematsu, K.; Nakamura, Y.; Nomura, M.; Shigemura, N.; Ninomiya, Y. Diurnal Variation of Sweet Taste Recognition Thresholds Is Absent in Overweight and Obese Humans. Nutrients 2018, 10, 297. https://doi.org/10.3390/nu10030297
Sanematsu K, Nakamura Y, Nomura M, Shigemura N, Ninomiya Y. Diurnal Variation of Sweet Taste Recognition Thresholds Is Absent in Overweight and Obese Humans. Nutrients. 2018; 10(3):297. https://doi.org/10.3390/nu10030297
Chicago/Turabian StyleSanematsu, Keisuke, Yuki Nakamura, Masatoshi Nomura, Noriatsu Shigemura, and Yuzo Ninomiya. 2018. "Diurnal Variation of Sweet Taste Recognition Thresholds Is Absent in Overweight and Obese Humans" Nutrients 10, no. 3: 297. https://doi.org/10.3390/nu10030297




