Effects of Cholesterol Levels on Mortality in Patients with Long-Term Peritoneal Dialysis Based on Residual Renal Function
Abstract
:1. Introduction
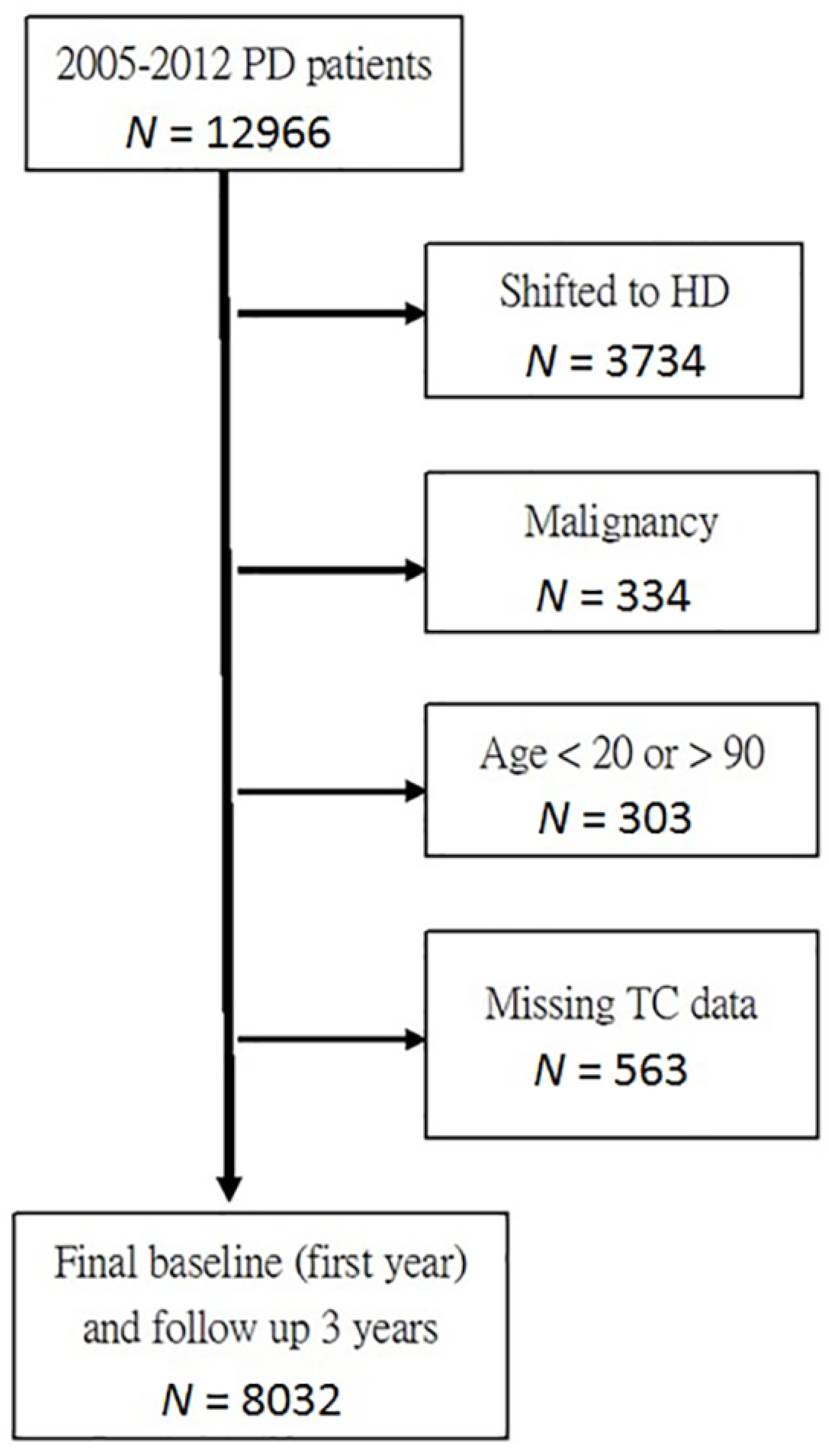
2. Materials and Methods
3. Results
3.1. Population Demographics
Baseline Characteristics of the Two Groups Categorized by Cholesterol Level
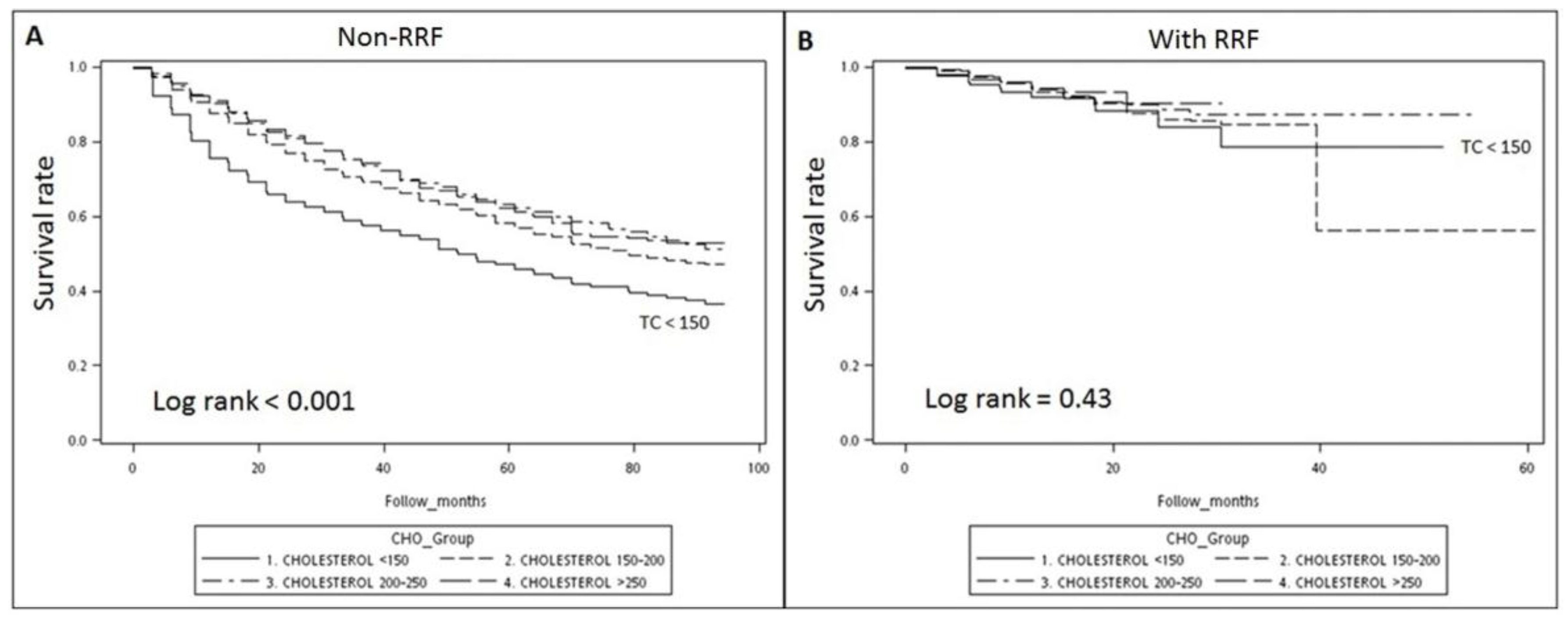
3.2. Survival Analysis: Kaplan–Meier Survival Curve
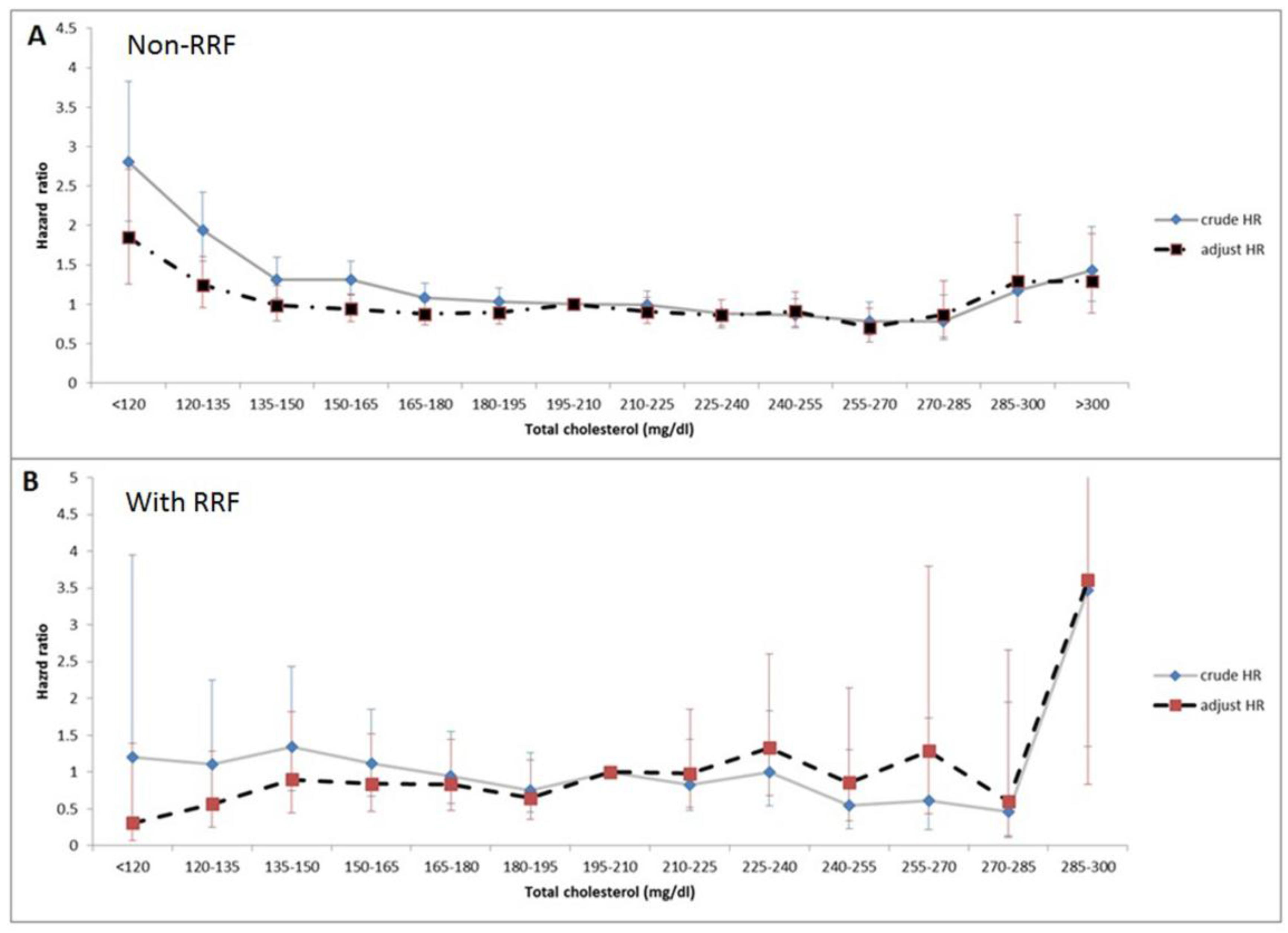
3.3. Association of Total Cholesterol Levels and Mortality with RRF Stratification
3.4. Subgroups Analysis with Stratification by the Existence of RRF
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shoji, T.; Abe, T.; Matsuo, H.; Egusa, G.; Yamasaki, Y.; Kashihara, N.; Shirai, K.; Kashiwagi, A.; Committee of Renal and Peripheral Arteries; Japan Atherosclerosis Society. Chronic kidney disease, dyslipidemia, and atherosclerosis. J. Atheroscler. Thromb. 2012, 19, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Mann, J.F.; Ruf, G.; Ritz, E.; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005, 353, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.N.; Baird, B.C.; Leypoldt, J.K.; Cheung, A.K.; Goldfarb-Rumyantzev, A.S. The association of lipid levels with mortality in patients on chronic peritoneal dialysis. Nephrol. Dial. Transplant. 2006, 21, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, Y.C.; Chen, H.H.; Chen, T.Z.; Hsu, C.C.; Peng, C.C.; Wu, M.S. Different effect of hypercholesterolemia on mortality in hemodialysis patients based on coronary artery disease or myocardial infarction. Lipids Health Dis. 2016, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Samouilidou, E.C.; Karpouza, A.P.; Kostopoulos, V.; Bakirtzi, T.; Pantelias, K.; Petras, D.; Tzanatou-Exarchou, H.J.; Grapsa, E. Lipid abnormalities and oxidized LDL in chronic kidney disease patients on hemodialysis and peritoneal dialysis. Ren. Fail. 2012, 34, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Phillips, L.; Russell, L.; Griffiths, A.; Russell, G.I.; Davies, S.J. Longitudinal lipid profiles on CAPD: Their relationship to weight gain, comorbidity, and dialysis factors. J. Am. Soc. Nephrol. 1998, 9, 1931–1939. [Google Scholar] [PubMed]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Leung, C.B.; Cheng, M.S.; Law, M.C.; Li, P.K. Metabolic syndrome in peritoneal dialysis patients: Choice of diagnostic criteria and prognostic implications. Clin. J. Am. Soc. Nephrol. 2014, 9, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.T.; Kao, T.W.; Chou, Y.H.; Wu, M.S.; Chen, Y.M.; Chuang, H.F.; Hung, K.Y.; Chu, T.S.; Wu, K.D.; Tsai, T.J. Associations of metabolic syndrome and its components with cardiovascular outcomes among non-diabetic patients undergoing maintenance peritoneal dialysis. Nephrol. Dial. Transplant. 2011, 26, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xiong, L.; Xu, Q.; Wu, J.; Huang, R.; Guo, Q.; Mao, H.; Yu, X.; Yang, X. Higher serum triglyceride to high-density lipoprotein cholesterol ratio was associated with increased cardiovascular mortality in female patients on peritoneal dialysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Oh, K.H.; Choi, K.H.; Kim, S.B.; Kim, Y.S.; Do, J.Y.; Kim, Y.L.; Kim, D.J. Statin therapy is associated with improved survival in incident peritoneal dialysis patients: Propensity-matched comparison. Nephrol. Dial. Transplant. 2011, 26, 4090–4094. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb-Rumyantzev, A.S.; Habib, A.N.; Baird, B.C.; Barenbaum, L.L.; Cheung, A.K. The association of lipid-modifying medications with mortality in patients on long-term peritoneal dialysis. Am. J. Kidney Dis. 2007, 50, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Sloand, J.A.; Li, P.K.; Story, K.; Bargman, J.M. Influence of low-glucose peritoneal dialysis on serum lipids and apolipoproteins in the IMPENDIA/EDEN trials. J. Clin. Lipidol. 2014, 8, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Perico, N.; Remuzzi, G.; Global Burden of Disease Study Genitourinary Disease Expert Group. High serum cholesterol: A missed risk factor for chronic kidney disease mortality. Lancet Diabetes Endocrinol. 2014, 2, 613–614. [Google Scholar] [CrossRef]
- Wanner, C.; Tonelli, M.; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 2004, 291, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
- Tokgoz, B. Clinical advantages of peritoneal dialysis. Perit. Dial. Int. 2009, 29 (Suppl. 2), S59–S61. [Google Scholar] [PubMed]
- Siamopoulos, K.C.; Elisaf, M.S.; Bairaktari, H.T.; Pappas, M.B.; Sferopoulos, G.D.; Nikolakakis, N.G. Lipid parameters including lipoprotein (a) in patients undergoing CAPD and hemodialysis. Perit. Dial. Int. 1995, 15, 342–347. [Google Scholar] [PubMed]
- Chen, H.Y.; Kao, T.W.; Huang, J.W.; Tsai, T.J.; Wu, K.D. Association between dyslipidemia and residual renal function in patients on chronic peritoneal dialysis. Clin. Nephrol. 2008, 70, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Fein, P.A.; Mittman, N.; Gadh, R.; Chattopadhyay, J.; Blaustein, D.; Mushnick, R.; Avram, M.M. Malnutrition and inflammation in peritoneal dialysis patients. Kidney Int. 2003, 64 (Suppl. 87), S87–S91. [Google Scholar] [CrossRef]
- Fellstrom, B.C.; Jardine, A.G.; Schmieder, R.E.; Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.W.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: Effects of case mix and the malnutrition-inflammation-cachexia syndrome. J. Am. Soc. Nephrol. 2007, 18, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Cobbe, S.M.; Ford, I.; Isles, C.G.; Lorimer, A.R.; MacFarlane, P.W.; McKillop, J.H.; Packard, C.J.; West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Engl. J. Med. 1995, 333, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Schatz, I.J.; Masaki, K.; Yano, K.; Chen, R.; Rodriguez, B.L.; Curb, J.D. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: A cohort study. Lancet 2001, 358, 351–355. [Google Scholar] [CrossRef]
| Variable | Whole Group | Non-RRF | With RRF | p Value |
|---|---|---|---|---|
| Number | 8032 | 5341 | 2691 | |
| Age (years) | 53.3 ± 14.8 | 53.1 ± 14.8 | 53.9 ± 14.6 | 0.014 |
| Male (%) | 3702 (46%) | 2365 (44%) | 1337 (50%) | <0.001 |
| DM (%) | 2918 (36%) | 1872 (35%) | 1046 (39%) | 0.001 |
| HTN (%) | 3893 (48%) | 2692 (50%) | 1201 (45%) | <0.001 |
| CHF (%) | 656 (8%) | 526 (10%) | 129 (5%) | <0.001 |
| LVH (%) | 578 (7%) | 492 (9%) | 86 (3%) | <0.001 |
| CVA (%) | 269 (3%) | 217 (4%) | 52 (2%) | <0.001 |
| HTN drugs (%) | 6548 (82%) | 4402 (82%) | 2146 (80%) | 0.004 |
| PD duration (years) (25th–75th (median)) | (0.79–1.06 (0.91)) | (0.82–1.31 (0.94)) | (0.64–0.95 (0.84)) | <0.001 |
| Following duration (years) | 2.11 ± 1.07 | 2.58 ± 0.88 | 1.17 ± 0.76 | <0.001 |
| Laboratory data | ||||
| WBC (x1000/uL) | 7.22 ± 2.27 | 7.30 ± 2.34 | 7.08 ± 2.12 | <0.001 |
| nPCR (gm/KgBW/day) | 1.04 ± 0.24 | 1.04 ± 0.24 | 1.04 ± 0.24 | 0.23 |
| Triglyceride (mg/dL) | 169.5 ± 110.0 | 174.7 ± 113.1 | 159.1 ± 102.8 | <0.001 |
| Glucose (mg/dL) | 125.8 ± 53.9 | 126.8 ± 56.6 | 123.7 ± 48.1 | 0.01 |
| Albumin (g/dL) | 3.69 ± 0.45 | 3.70 ± 0.45 | 3.65 ± 0.45 | <0.001 |
| Hct (%) | 30.72 ± 3.67 | 30.48 ± 3.73 | 31.19 ± 3.48 | <0.001 |
| Ca (mg/dL) | 9.09 ± 0.76 | 9.17 ± 0.77 | 8.93 ± 0.72 | <0.001 |
| P (mg/dL) | 5.05 ± 1.13 | 5.02 ± 1.13 | 5.11 ± 1.12 | 0.001 |
| ALK-P (u/L) | 118.0 ± 98.9 | 121.4 ± 100.5 | 111.2 ± 95.2 | <0.001 |
| i-PTH (pg/mL) | 269.4 ± 206.6 | 261.3 ± 206.3 | 285.3 ± 206.1 | <0.001 |
| Ca*P | 45.75 ± 11.12 | 45.89 ± 11.39 | 45.47 ± 10.56 | 0.11 |
| Non-RRF (n = 5341) | With RRF (n = 2691) | |||||
|---|---|---|---|---|---|---|
| n (%) | Crude HR (95% CI) | Adjust HR (95% CI) | n (%) | Crude HR (95% CI) | Adjust HR (95% CI) | |
| Cholesterol (mg/dL) | ||||||
| <120 | 65 (1.2%) | 2.81 (2.01–3.83) ** | 1.85 (1.26–2.71) * | 45 (1.7%) | 1.21 (0.37-3.95) | 0.31 (0.07–1.59) |
| 120–135 | 164 (3.1%) | 1.94 (1.55–2.43) ** | 1.24 (0.96–1.60) | 111 (4.1%) | 1.10 (0.54–2.25) | 0.56 (0.25–1.29) |
| 135–150 | 302 (5.7%) | 1.32 (1.08–1.60) * | 0.99 (0.79–1.23) | 178 (6.6%) | 1.35 (0.74–2.43) | 0.90 (0.45–1.82) |
| 150–165 | 533 (10.0%) | 1.31 (1.12–1.55) * | 0.94 (0.78–1.13) | 330 (12.3%) | 1.11 (0.67–1.86) | 0.84 (0.47–1.53) |
| 165–180 | 728 (13.6%) | 1.09 (0.93–1.27) | 0.88 (0.74–1.04) | 408 (15.2%) | 0.95 (0.58–1.55) | 0.83 (0.48–1.44) |
| 180–195 | 801 (15.0%) | 1.04 (0.89–1.20) | 0.90 (0.76–1.06) | 431 (16.0%) | 0.76 (0.45–1.26) | 0.65 (0.36–1.17) |
| 195–210 | 848 (15.9%) | Ref. | Ref. | 361 (13.4%) | Ref. | Ref. |
| 210–225 | 709 (13.3%) | 1.00 (0.85–1.17) | 0.91 (0.76–1.09) | 300 (11.1%) | 0.83 (0.48–1.45) | 0.98 (0.52–1.86) |
| 225–240 | 475 (8.9%) | 0.88 (0.74–1.06) | 0.86 (0.70–1.05) | 218 (8.1%) | 1.00 (0.55–1.83) | 1.3 (0.68–2.60) |
| 240–255 | 311 (5.8%) | 0.87 (0.71–1.07) | 0.91 (0.72–1.15) | 135 (5.0%) | 0.54 (0.23–1.30) | 0.86 (0.34–2.14) |
| 255–270 | 189 (3.5%) | 0.79 (0.61–1.02) | 0.71 (0.52–0.95) * | 77 (2.9%) | 0.61 (0.22–1.74) | 1.29 (0.44–3.80) |
| 270–285 | 97 (1.8%) | 0.78 (0.55–1.12) | 0.87 (0.58–1.30) | 49 (1.8%) | 0.47 (0.11–1.95) | 0.61 (0.14–2.66) |
| 285–300 | 46 (0.9%) | 1.17 (0.77–1.79) | 1.29 (0.78–2.14) | 26 (1.0%) | 3.47 (1.35–8.93) * | 3.61 (0.83–15.70) |
| >300 | 73 (1.4%) | 1.43 (1.04–1.98) * | 1.30 (0.89–1.90) | 22 (0.8%) | 0 | 0 |
| Group | Non-RRF (n = 5,341) | With RRF (n = 2691) | ||||
|---|---|---|---|---|---|---|
| Variables | n (%) | Crude HR (95% CI) | Adjusted HR (95% CI) | n (%) | Crude HR (95% CI) | Adjusted HR (95% CI) |
| Cholesterol (mg/dL) | ||||||
| <150 | 525 (10%) | 1.46 (1.28–1.66) ** | 1.23 (1.05–1.43) ** | 328 (12%) | 1.22 (0.81–1.85) | 0.83 (0.52–1.35) |
| 150–200 | 2329 (44%) | Ref. | Ref. | 1289 (48%) | Ref. | Ref. |
| 200–250 | 1986 (37%) | 0.85 (0.78–0.94) ** | 0.98 (0.88–1.09) | 869 (32%) | 0.88 (0.64–1.02) | 1.28 (0.89–1.84) |
| >250 | 501 (9%) | 0.86 (0.74–1.00) * | 1.03 (0.86–1.23) | 205 (8%) | 0.79 (0.43–1.43) | 1.16 (0.57–2.36) |
| DM | ||||||
| Y | 1872 (35%) | 1.82 (1.68–1.98) ** | 1.53 (1.38–1.70) ** | 1046 (39%) | 1.85 (1.37–2.38) | 1.35 (0.96–1.90) |
| N | 3469 (65%) | Ref. | Ref. | 1645 (61%) | Ref. | Ref. |
| HTN | ||||||
| Y | 2692 (50%) | 0.53 (0.48–0.57) ** | 0.77 (0.70–0.86) ** | 1201 (45%) | 0.91 (0.69–1.20) | 1.10 (0.78–1.55) |
| N | 2649 (50%) | Ref. | Ref. | 1490 (55%) | Ref. | Ref. |
| Age | ||||||
| ≤50 | 2342 (44%) | 0.50 (0.46–0.54) ** | 0.71 (0.64–0.80) ** | 1075 (40%) | 0.51 (0.38–0.70) ** | 0.81 (0.55–1.19) |
| >50 | 2999 (56%) | Ref. | Ref. | 1616 (60%) | Ref. | Ref. |
| Sex | ||||||
| Male | 2365 (44%) | Ref. | Ref. | 1337 (50%) | Ref. | Ref. |
| Female | 2976 (56%) | 0.84 (0.77–0.91) ** | 0.81 (0.73–0.90) ** | 1354 (50%) | 0.87 (0.66–1.14) | 0.71 (0.50–0.99) * |
| Non-RRF (n = 5341) | The 3rd Year | |||
| Low | Normal | High | ||
| The 1st Year | ||||
| Low | 1.73 (1.50–1.99) ** | 0.55 (0.38–0.78) ** | – | |
| Normal | 0.89 (0.66–1.21) | Ref. | 0.65 (0.52–0.81) ** | |
| High | – | 0.65 (0.55–0.77) ** | 0.85 (0.77–0.93) ** | |
| With RRF (n = 2691) | The 3rd Year | |||
| Low | Normal | High | ||
| The 1st Year | ||||
| Low | 1.33 (0.86–2.05) | 0.36 (0.09–1.48) | – | |
| Normal | 0.44 (0.11–1.76) | Ref. | 0.29 (0.09–0.92) * | |
| High | – | 0.34 (0.14–0.83) * | 0.89 (0.65–1.21) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Lin, Y.-C.; Peng, C.-C.; Chen, K.-C.; Chen, H.-H.; Fang, T.-C.; Sung, S.-Y.; Wu, M.-S. Effects of Cholesterol Levels on Mortality in Patients with Long-Term Peritoneal Dialysis Based on Residual Renal Function. Nutrients 2018, 10, 300. https://doi.org/10.3390/nu10030300
Lin Y-C, Lin Y-C, Peng C-C, Chen K-C, Chen H-H, Fang T-C, Sung S-Y, Wu M-S. Effects of Cholesterol Levels on Mortality in Patients with Long-Term Peritoneal Dialysis Based on Residual Renal Function. Nutrients. 2018; 10(3):300. https://doi.org/10.3390/nu10030300
Chicago/Turabian StyleLin, Yen-Chung, Yi-Chun Lin, Chiung-Chi Peng, Kuan-Chou Chen, Hsi-Hsien Chen, Te-Chao Fang, Shian-Ying Sung, and Mai-Szu Wu. 2018. "Effects of Cholesterol Levels on Mortality in Patients with Long-Term Peritoneal Dialysis Based on Residual Renal Function" Nutrients 10, no. 3: 300. https://doi.org/10.3390/nu10030300





