Selenium Accumulation, Antioxidant Enzyme Levels, and Amino Acids Composition in Chinese Mitten Crab (Eriocheir sinensis) Fed Selenium-Biofortified Corn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Diets and Design
2.3. Sample Collection, Preparation, and Storage
2.4. Determination of the Total Selenium Level
2.5. Measurement of Hemolymph Supernatant Antioxidant Enzyme Activities
2.6. Analysis of Amino Acids, Protein, and Crude Fat
2.7. Data Analysis
3. Results
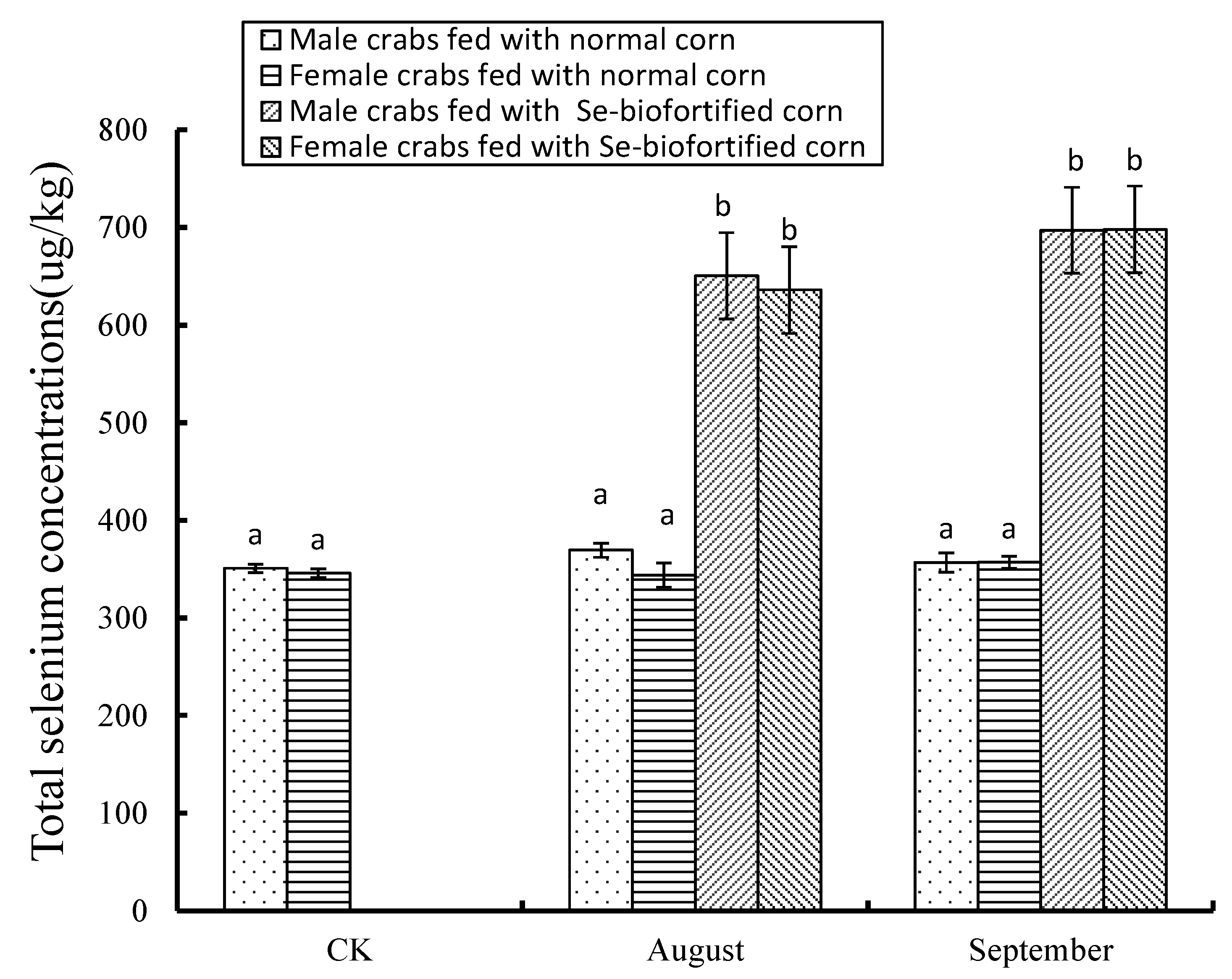
3.1. Effects of Se-Biofortified Corn on the Total Se Level in Crabmeat
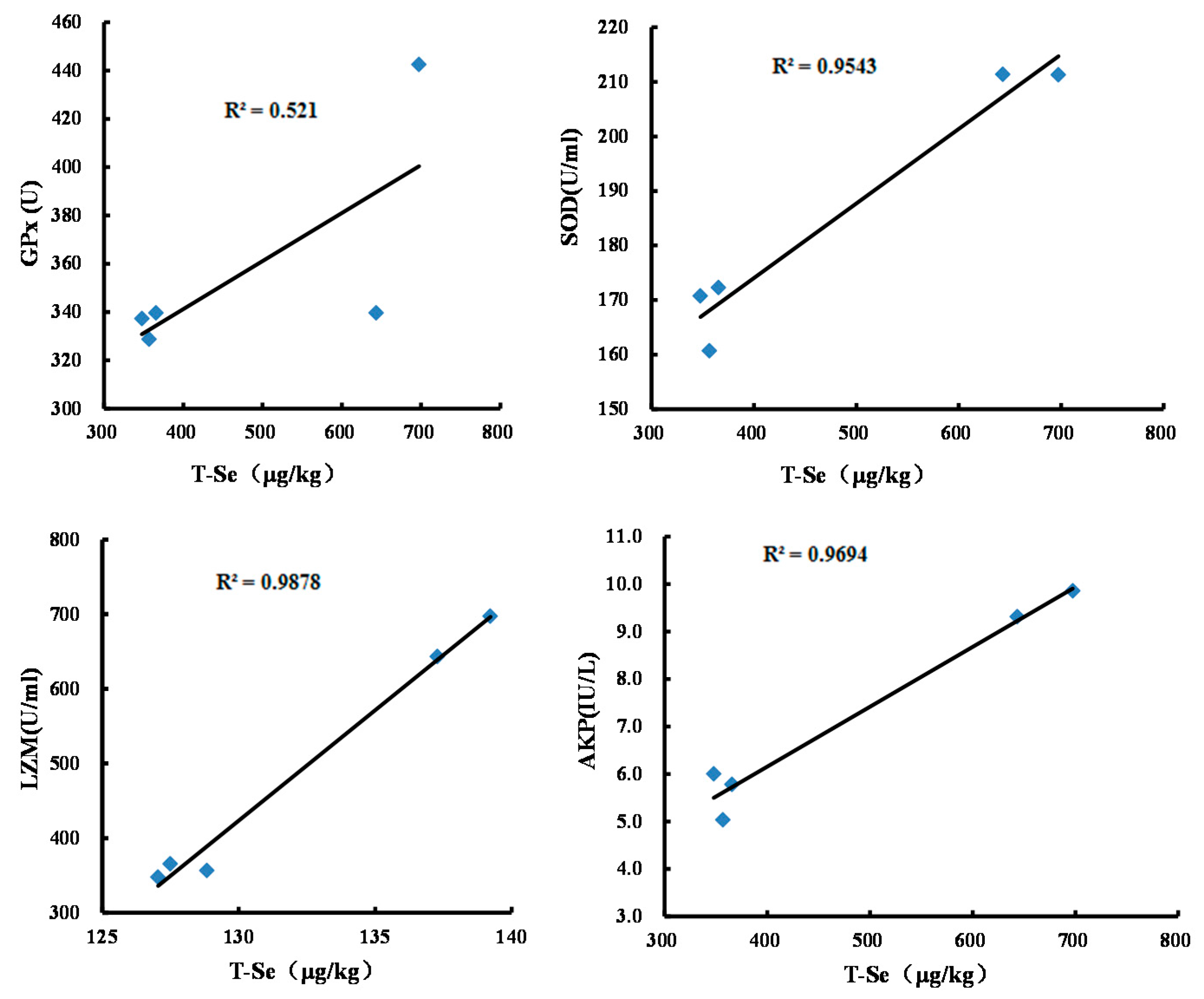
3.2. Effects of Se-Biofortified Corn on Hemolymph Supernatant Antioxidant Enzyme Activities
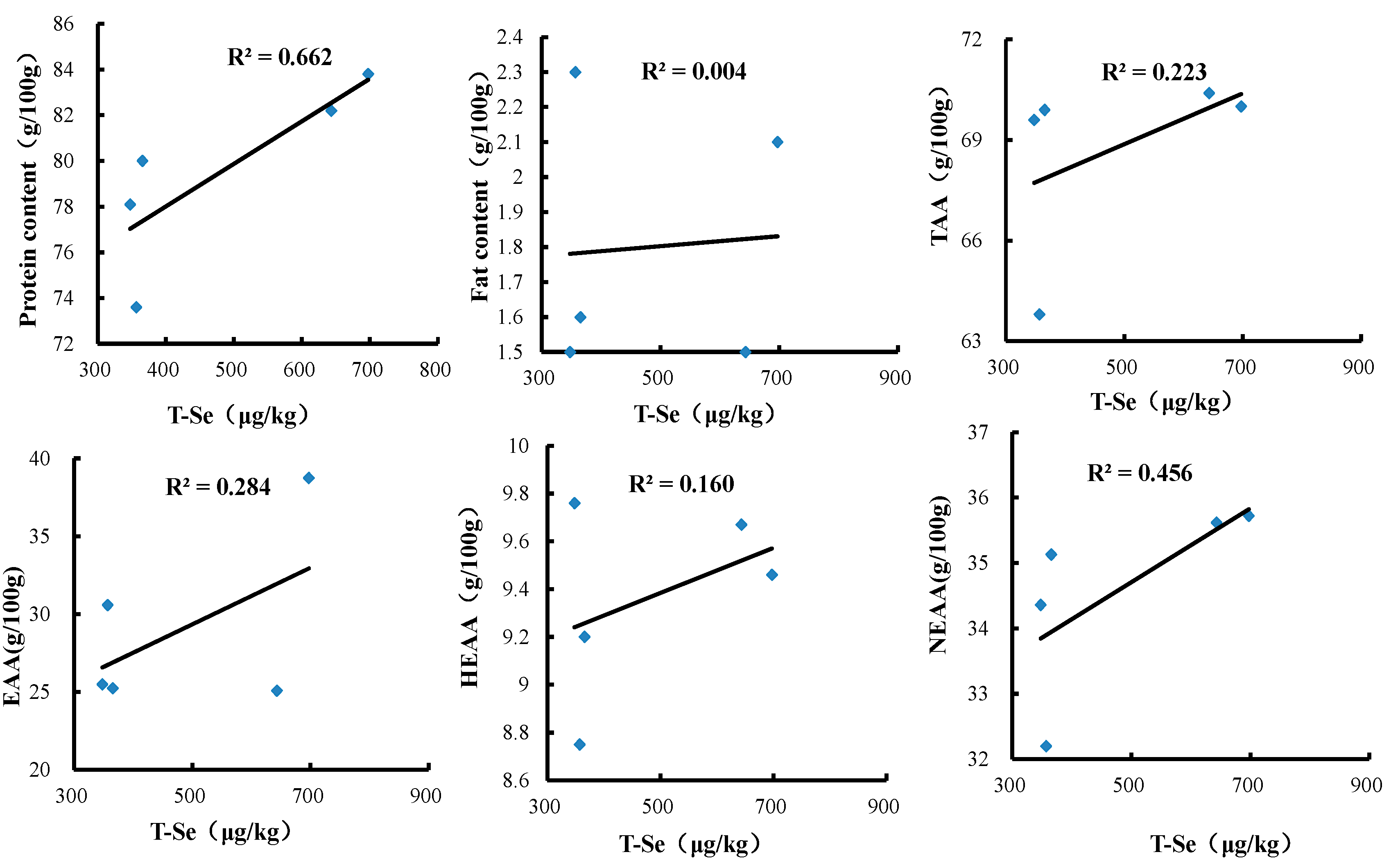
3.3. Effects of Se-Biofortified Corn on the Contents of Protein, Crude Fats, and Amino Acids in Crabmeat
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, K.; Li, E.; Yu, N.; Du, Z.; Chen, L. Biochemical composition and nutritional value analysis of Chinese mitten crab, Eriocheir sinensis, grown in pond. Glob. Adv. Res. J. Agric. Sci. 2013, 2, 164–173. [Google Scholar]
- Zou, G.Z.; Sun, H.S. Research Advances and Prospect in Aquatic Crustaceans Immunology. Life Sci. Instrum. 2009, 6, 17–21. (In Chinese) [Google Scholar]
- Jin, Z.W.; Zheng, Z.M.; Wu, S.J.; You, E.M.; Hu, A.K. Preliminary study on improvement of pond water quality by bottom aeration. South China Fish. Sci. 2010, 6, 20–25. [Google Scholar]
- Fu, J.J.; Liu, X.L.; Wu, C.Y.; Li, L.; Cao, J.M. Effects of selenium on antioxidation and immunity in aquatic animals. Guangdong Agric. Sci. 2013, 40, 124–126. (In Chinese) [Google Scholar]
- Rotruck, J.T.; Hoekstra, W.G.; Pope, A.L. Glucose-dependent Protection by Dietary Selenium against Haemolysis of Rat Erythrocytes in vitro. Nat. New Biol. 1971, 231, 223–224. [Google Scholar] [CrossRef]
- Poston, H.A.; Combs, G.F., Jr.; Leibovitz, L. Vitamin E and selenium interrelations in the diet of Atlantic salmon (Salmo salar): Gross, histological and biochemical deficiency signs. J. Nutr. 1976, 106, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; Pirie, B.J.S.; Adron, J.W.; Cowey, C.B. Some effects of selenium deficiency on glutathione peroxidase (EC 1.11.1.9) activity and tissue pathology in rainbow trout (Salmo gairdneri). Br. J. Nutr. 1986, 55, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Xu, H.M.; Xiao, G.H.; Zhao, C.L.; Wang, Z.H.; Cai, D.B.; Li, H.Q.; Zhao, J.H. Effects of Selenium on the Antioxidant Enzymes Response of Neocaridina heteropoda Exposed to Ambient Nitrite. Bull. Environ. Contam. Toxicol. 2010, 84, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Shi, M.; Yuan, H.; Yuan, L.; Lu, W.; Zhang, J.; Tong, J.; Song, X. Dietary nano-selenium relieves hypoxia stress and, improves immunity and disease resistance in the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2016, 54, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lovell, R.T.; Klesius, P.H. Response to Edwardsiella ictaluri challenge by Channel catfish fed organic and inorganic sources of selenium. J. Aquat. Anim. Health 1997, 9, 172–179. [Google Scholar] [CrossRef]
- Bell, J.G.; Cowey, C.B. Digestibility and bioavailability of dietary selenium from fishmeal, selenite, selenomethionine and selenocystine in Atlantic salmon (Salmo salar). Aquaculture 1989, 81, 61–68. [Google Scholar] [CrossRef]
- Wang, C.; Lovell, R.T. Organic selenium sources, selenomethionine and selenoyeast, have higher bioavailability than an inorganic selenium source, sodium selenite, in diets for channel catfish (Ictalurus punctatus). Aquaculture 1997, 152, 223–234. [Google Scholar] [CrossRef]
- Le, K.T.; Fotedar, R. Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquaculture 2014, 420–421, 57–62. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, Z.X.; Zhang, K.; Zhang, Y.M.; Jiang, Y.S.; Liu, C.B.; Wu, Y.Q. Effects of dietary selenomethionine levels on growth and some immune indices in juvenile sea cucumber Apostichopus japonicus. J. Dalian Ocean Univ. 2012, 27, 110–115. (In Chinese) [Google Scholar]
- Malagoli, M.; Schiavon, M.; dall’Acqua, S.; Pilon-Smith, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Ranches, J.; Vendramini, J.M.B.; Arthington, J.D. Effects of selenium biofortification of hayfields on measures of selenium status in cows and calves consuming these forages. J. Anim. Sci. 2016, 95, 120–128. [Google Scholar] [CrossRef]
- Shi, L.; Ren, Y.S.; Zhang, C.X.; Yue, W.B.; Lei, F.L. Effects of organic selenium (selenium-enriched yeast) supplementation in gestation diet on antioxidant status, hormone profile and haemato-biochemical parameters in Taihang Black Goats. Anim. Feed Sci. Technol. 2018, 238, 57–65. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Xu, W.J.; Xu, Y.; Wu, D.; Sun, Y.H.; Deng, F.; Shen, W.L. Amino acid adsorption on mesoporous materials: Influence of type of amino acids, modification of mesoporous materials, and solution condition. J. Phys. Chem. B 2008, 112, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Wang, J.Q.; Xu, W.D.; Han, J.B.; Zhou, Z.C.; Dong, Y. Amino acid compositions in Gonad of Sea Urchin Strongylocentrotus intermedius. Fish. Sci. 2008, 12, 619–621. (In Chinese) [Google Scholar]
- Borges, A.; Scotti, L.V.; Siqueira, D.R.; Zanini, R.; Amaral, F.D.; Jurinitz, D.F.; Wassermann, G.F. Changes in hematological and supernatant biochemical values in jundiá Rhamdia quelen due to sub-lethal toxicity of cypermethrin. Chemosphere 2007, 69, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Sudová, E.; Piačková, V.; Kroupová, H.; Pijáček, M.; Svobodová, Z. The effect of praziquantel applied per os on selected haematological and biochemical indices in common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2009, 35, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Velisek, J.; Zlabek, V.; Grabic, R.; Machova, J.; Kolarova, J.; Randak, T. Hepatic antioxidant status and hematological parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chem.-Biol. Interact. 2010, 183, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Dı́Az-Salvago, E.; Bell, J.G.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Yang, K.; Metcalf, W.W. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. Proc. Natl. Acad. Sci. USA 2004, 101, 7919–7924. [Google Scholar] [CrossRef] [PubMed]
- Pacitti, D.; Wang, T.; Page, M.M.; Martin, S.A.M.; Sweetman, J.; Feldmann, J.; Secombes, C.J. Characterization of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase genes in rainbow trout (Oncorhynchus mykiss) and their modulation by in vitro selenium exposure. Aquat. Toxicol. 2013, 130–131, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Li, W.; Xu, Z. Effect of different selenium source on growth performances, glutathione peroxidase activities, muscle composition and selenium concentration of allogynogenetic crucian carp (Carassius auratus gibelio). Anim. Feed Sci. Technol. 2007, 134, 243–251. [Google Scholar] [CrossRef]
- Wang, H.W.; Cai, D.B.; Xiao, G.H.; Zhao, C.L.; Wang, Z.H.; Xu, H.M.; Guan, Y.Q. Effects of selenium on the activity of antioxidant enzymes in the shrimp, Neocaridina heteropoda. Isr. J. Aquac.–Bamidgeh 2009, 61, 322–329. [Google Scholar]
- Avanzo, J.L.; Mendonça, C.X.D.; Pugine, S.M.P.; Cesar, M.D.C. Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2001, 129, 163–173. [Google Scholar] [CrossRef]
- Low, K.W.; Sin, Y.M. In vivo and in vitro effects of mercuric chloride and sodium selenite on some non-specific immune responses of blue gourami, Trichogaster trichopterus (Pallus). Fish Shellfish Immunol. 1996, 6, 351–362. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, J.; Huang, Y.; Wang, G.; Wenyan, M.O.; Zhou, T.; Sun, Z.; Liu, X. The combined effects of β-glucan with selenium and vitamin E on the growth performance, supernatant immune and antioxidant indexes, and disease resistance of Litopenaeus vannamei. J. Fish. Sci. China 2013, 20, 997–1006. [Google Scholar]
- Le, K.T.; Fotedar, R.; Partridge, G. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (Seriola lalandi): Physiological and immune responses. Aquac. Nutr. 2014, 20, 303–313. [Google Scholar] [CrossRef]
- Li, J.Y.; Sun, X.; Zheng, F.; Sun, H. Histochemical localization and characterization of AKP, ACP, NSE, and POD from cultured Apostichopus japonicus. Chin. J. Oceanol. Limnol. 2009, 27, 550–554. (In Chinese) [Google Scholar]
- Wei, G.L.; Ming, K.W.; Ni, C.; Sin, Y.M. Effect of combined copper, zinc, chromium and selenium by orthogonal array design on alkaline phosphatase activity in liver of the red sea bream, Chrysophrys major. Aquaculture 1995, 131, 219–230. [Google Scholar]
- Regoli, F.; Principato, G. Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: Implications for the use of biochemical biomarkers. Aquat. Toxicol. 1995, 31, 143–164. [Google Scholar] [CrossRef]
- Mongkolsuk, S.; Whangsuk, W.; Vattanaviboon, P.; Loprasert, S.; Fuangthong, M. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 2000, 182, 6845–6849. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.Q.; Chai, T.Y.; Zhang, Y.X.; Xu, J.; Wei, W. Enhancement of Cd tolerance in transgenic tobacco plants overexpressing a Cd-induced catalase cDNA. Chemosphere 2009, 76, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; Nicol, F.; Beckett, G.J. Hepatic iodothyronine 5′-deiodinase. The role of selenium. Biochem. J. 1990, 272, 537–540. [Google Scholar] [PubMed]
- Hicks, B.; Hilton, J.; Ferguson, H. Influence of dietary selenium on the occurrence of nephrocalcinosis in the rainbow trout, Salmo gairdneri Richardson. J. Fish Dis. 1984, 7, 379–389. [Google Scholar] [CrossRef]
- Li, X.X.; Chen, F.; Pan, Q.; Wang, L.; Feng, T.; Gan, L.; Jinghua, F.U.; Cao, J. Effects of dietary selenium sources on growth, body composition and antioxidant performance of Juvenile Pacific White Leg Shrimp Litopenaeus vannamei. Fish. Sci. 2016, 3, 199–203. (In Chinese) [Google Scholar]
- Waschulewski, I.H.; Sunde, R.A. Effect of dietary methionine on utilization of tissue selenium from dietary selenomethionine for glutathione peroxidase in the rat. J. Nutr. 1988, 118, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Qin, F.J.; Yuan, L.X.; Song, X.H.; Bu, Y.X.; Liu, Z.J.; Fu, J.G. Effects of nano-Se on growth performance, selenium content and nutrient composition of Chinese mitten crabs (Eriocheir sinensis). Feed Ind. 2015, 36, 21–25. (In Chinese) [Google Scholar]
- Ashouri, S.; Keyvanshokooh, S.; Salati, A.P.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, hemolymph biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Tian, W.J.; Li, E.C.; Chen, L.Q.; Sun, L.M.; Chen, Y.L.; Li, M.; Jiang, X.; Du, Z.Y. Growth, body composition and anti-oxidative status of juvenile Chinese mitten crabs, Eriocheir sinensis fed different dietary selenium levels. J. Fish. Sci. China 2014, 1, 92–100. (In Chinese) [Google Scholar]

| Item | July Sample (CK) | August Sample | September Sample | ||
|---|---|---|---|---|---|
| Normal Corn | Se-Biofortified Corn | Normal Corn | Se-Biofortified Corn | ||
| g·kg−1 | |||||
| Protein | 7.81 | 8.00 | 8.22 | 7.36 | 8.38 |
| Crude fat | 0.15 | 0.16 | 0.15 | 0.23 | 0.21 |
| Lys 1 | 0.54 | 0.55 | 0.54 | 0.49 | 0.54 |
| Phe 1,3 | 0.35 | 0.32 | 0.32 | 0.29 | 0.32 |
| Mer 1 | 0.21 | 0.21 | 0.21 | 0.21 | 0.22 |
| Thr 1 | 0.36 | 0.36 | 0.35 | 0.32 | 0.35 |
| Ile 1 | 0.26 | 0.25 | 0.25 | 0.23 | 0.24 |
| Leu 1 | 0.57 | 0.57 | 0.57 | 0.5.2 | 0.56 |
| Val 1 | 0.27 | 0.26 | 0.26 | 0.23 | 0.25 |
| His 2 | 0.24 | 0.23 | 0.23 | 0.21 | 0.23 |
| Arg 2 | 0.74 | 0.69 | 0.74 | 0.67 | 0.72 |
| Glu 3,4 | 1.07 | 1.10 | 1.09 | 1.01 | 1.08 |
| Gly 3,4 | 0.33 | 0.39 | 0.42 | 0.33 | 0.42 |
| Ala 3,4 | 0.57 | 0.59 | 0.60 | 0.55 | 0.62 |
| Asp 3,4 | 0.70 | 0.70 | 0.69 | 0.64 | 0.6.9 |
| Tyr 3,4 | 0.38 | 0.36 | 0.35 | 0.32 | 0.35 |
| Pro 4 | 0.09 | 0.19 | 0.11 | 0.09 | 0.12 |
| Ser 4 | 0.30 | 0.3 | 0.31 | 0.28 | 0.30 |
| ∑EAA | 2.55 | 2.52 | 2.51 | 3.06 | 3.87 |
| ∑HEAA | 0.98 | 0.92 | 0.97 | 0.88 | 0.95 |
| ∑NEAA | 3.44 | 3.51 | 3.56 | 3.22 | 3.57 |
| ∑DAA | 3.40 | 3.13 | 3.15 | 2.85 | 3.15 |
| ∑TAA | 6.96 | 7.00 | 7.04 | 6.38 | 0.70 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Zhang, R.; Ma, X.; Yang, L.; Zheng, Q.; Chen, D.; Li, M.; Fan, T.; Liu, Y.; Pan, L.; et al. Selenium Accumulation, Antioxidant Enzyme Levels, and Amino Acids Composition in Chinese Mitten Crab (Eriocheir sinensis) Fed Selenium-Biofortified Corn. Nutrients 2018, 10, 318. https://doi.org/10.3390/nu10030318
Yuan L, Zhang R, Ma X, Yang L, Zheng Q, Chen D, Li M, Fan T, Liu Y, Pan L, et al. Selenium Accumulation, Antioxidant Enzyme Levels, and Amino Acids Composition in Chinese Mitten Crab (Eriocheir sinensis) Fed Selenium-Biofortified Corn. Nutrients. 2018; 10(3):318. https://doi.org/10.3390/nu10030318
Chicago/Turabian StyleYuan, Linxi, Ru Zhang, Xuzhou Ma, Ling Yang, Qing Zheng, Dong Chen, Miao Li, Ting Fan, Yongxian Liu, Liping Pan, and et al. 2018. "Selenium Accumulation, Antioxidant Enzyme Levels, and Amino Acids Composition in Chinese Mitten Crab (Eriocheir sinensis) Fed Selenium-Biofortified Corn" Nutrients 10, no. 3: 318. https://doi.org/10.3390/nu10030318




