Resveratrol in Patients with Minimal Hepatic Encephalopathy
Abstract
:1. Introduction
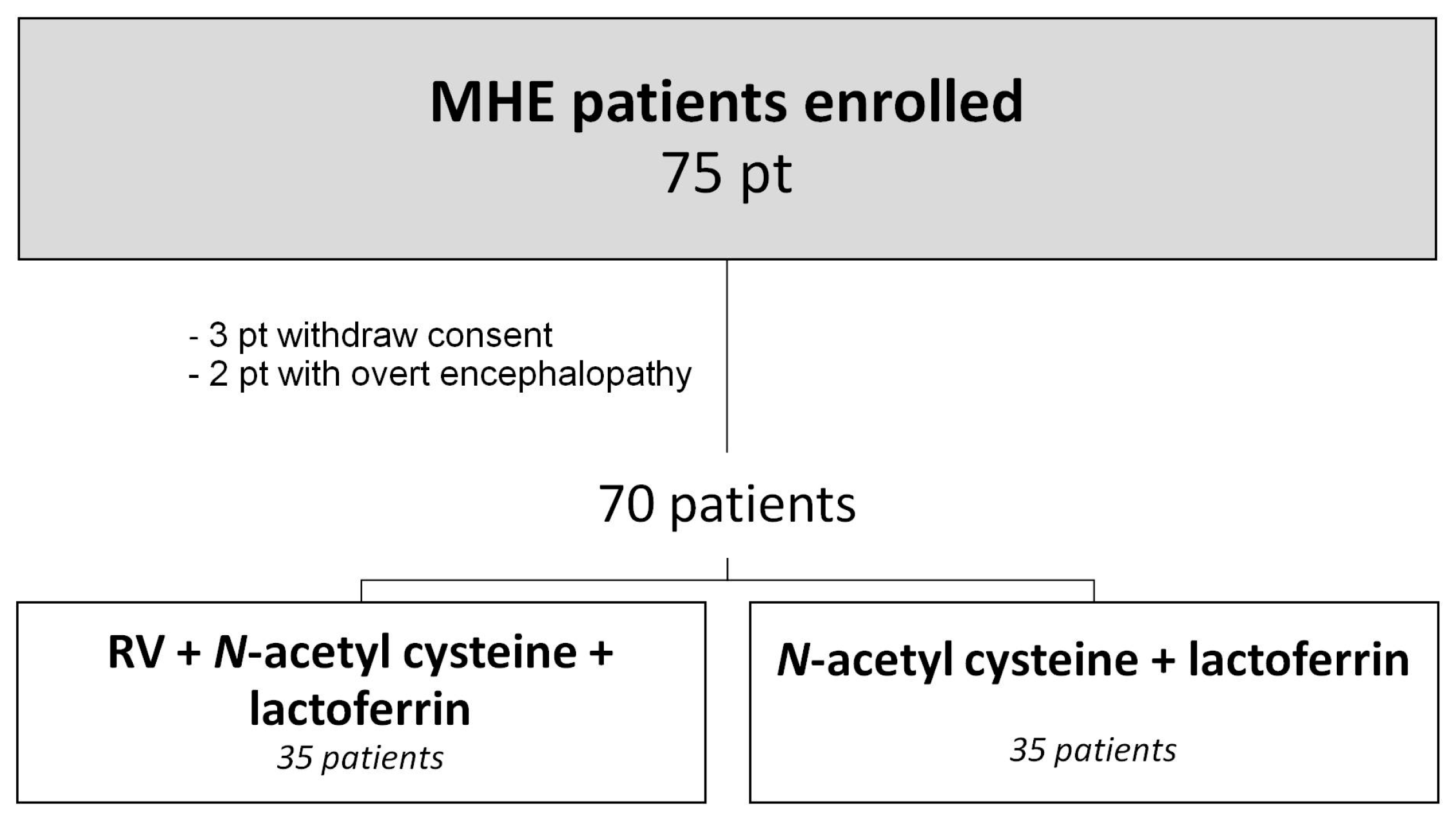
2. Patients and Methods
2.1. Study Design
2.2. Protocol
2.2.1. Diagnosis of MHE
2.2.2. Neuropsychological Testing
PHES Test Battery
2.2.3. SF-36 (Short Form—36 Health Survey)
2.2.4. Beck Depression Inventory (BDI)
2.2.5. State-Trait Anxiety Inventory (STAI)
2.2.6. Clinical and Laboratory Assessment
2.2.7. Neurological Assessment
2.2.8. Efficacy and Tolerability Assessment
2.3. Statistical Analysis
3. Results
3.1. Effects of RV on Biohumoral Findings
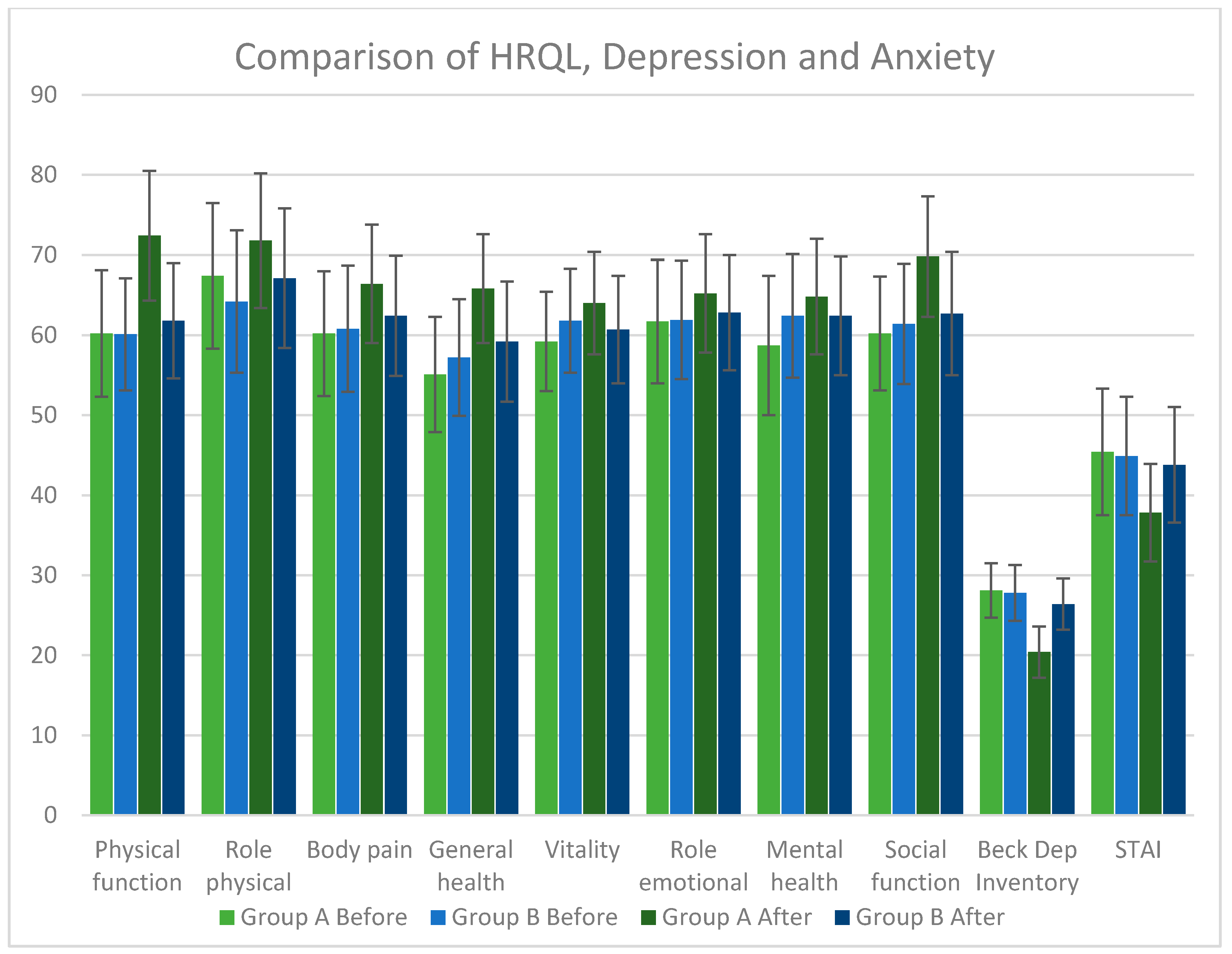
3.2. Effects of RV in Depression, in Anxiety and in Quality of Life
3.3. Adverse Events
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malaguarnera, M.; Vacante, M.; Bertino, G.; Neri, S.; Malaguarnera, M.; Gargante, M.P.; Motta, M.; Lupo, L.; Chisari, G.; Bruno, C.M.; et al. The supplementation of acetyl-l-carnitine decreases fatigue and increases quality of life in patients with hepatitis C treated with pegylated interferon-α 2b plus ribavirin. J. Interferon Cytokine Res. 2011, 31, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.H.; Stine, J.G. Review article: Prescribing medications in patients with cirrhosis—A practical guide. Aliment. Pharmacol. Ther. 2013, 37, 1132–1156. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.; Krutmann, J.; Li, Y.-H.; McDaniel, D.; Krol, Y. Resveratrol: A unique antioxidant offering a multi-mechanistic approach for treating aging skin. J. Drugs Dermatol. 2013, 12, 1389–1394. [Google Scholar] [PubMed]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Campagna, M.; Rivas, C. Antiviral activity of resveratrol. Biochem. Soc. Trans. 2010, 38, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorg. Med. Chem. Lett. 2012, 22, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-H.; Zang, N.; Li, S.; Wang, L.; Deng, Y.; He, Y.; Yang, X.Q.; Liu, E.M. Resveratrol Inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation 2012, 35, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R.S. Recent advances of resveratrol in nanostructured based delivery systems and in the management of HIV/AIDS. J. Control Release 2014, 194, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Aluyen, J.K.; Ton, Q.N.; Tran, T.; Yang, A.E.; Gottlieb, H.B.; Bellanger, R.A. Resveratrol: Potential as anticancer agent. J. Diet. Suppl. 2012, 9, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Ren, J. From French Paradox to cancer treatment: Anti-cancer activities and mechanisms of resveratrol. Anti-Cancer Agents Med. Chem. 2014, 14, 806–825. [Google Scholar] [CrossRef]
- Pennisi, M.; Bertino, G.; Gagliano, C.; Malaguarnera, M.; Bella, R.; Borzì, A.M.; Madeddu, R.; Malaguarnera, G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients 2017, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Magaji, M.G.; Iniaghe, L.O.; Abolarin, M.; Abdullahi, O.I.; Magaji, R.A. Neurobehavioural evaluation of resveratrol in murine models of anxiety and schizophrenia. Metab. Brain Dis. 2017, 32, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Conn, H.O. A clinical hepatologist’s predictions about non-absorbed carbohydrates for the early twenty-first century. Scand. J. Gastroenterol. 1997, 222, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. In The Liver and Portal Hypertension; Child, C.G., Ed.; Saunders: Philadelphia, PA, USA, 1964; pp. 50–64. [Google Scholar]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy—Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Snow, K.K.; Gandek, B. SF-36 Health Survey: Manual and Interpretation Guide; The Health Institute, New England Medical Center: Boston, MA, USA, 1993. [Google Scholar]
- Bonkovsky, H.L.; Woolley, J.M. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy. The Consensus Interferon Study Group. Hepatology 1999, 29, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R. Hepatitis C virus infection: Quality of life and side effects of treatment. J. Hepatol. 1999, 31, 250–254. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Steer, R.; Garbin, M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 122–132. [Google Scholar] [CrossRef]
- Spielberger, C.D. Manual for the State—Trait Anxiety Inventory: STAI (Form Y); Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Da Fonseca-Wollheim, F. Direct determination of plasma ammonia without deproteinization. An improved enzymic determination of ammonia, II (author’s transl). Z. Klin. Chem. Klin. Biochem. 1973, 11, 426–431. [Google Scholar] [PubMed]
- Latteri, S.; Malaguarnera, G.; Mannino, M.; Pesce, A.; Currò, G.; Tamburrini, S.; Scuderi, M. Ultrasound as point of care in management of polytrauma and its complication. J. Ultrasound 2017, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Flamm, S.L. Covert Hepatic Encephalopathy: Who Should Be Tested and Treated? Clin. Liver Dis. 2015, 19, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Latteri, S.; Catania, V.E.; Malaguarnera, M. Reduction of cardiovascular risk in subjects with high lipoprotein (a) levels. J. Thorac. Dis. 2017, 9, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Catania, V.E.; Francaviglia, A.; Malaguarnera, M.; Drago, F.; Motta, M.; Latteri, S. Lipoprotein (a) in patients with hepatocellular carcinoma and portal vein thrombosis. Aging Clin. Exp. Res. 2017, 29, 185–190. [Google Scholar] [CrossRef] [PubMed]
- La Verde, M.; Mule, S.; Zappala, G.; Privitera, G.; Maugeri, G.; Pecora, F.; Marranzano, M. Higher adherence to the Mediterranean diet is inversely associated with having hypertension: Is low salt intake a mediating factor? Int. J. Food Sci. Nutr. 2018, 69, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Platania, A.; Zappala, G.; Mirabella, M.U.; Gullo, C.; Mellini, G.; Beneventano, G.; Maugeri, G.; Marranzano, M. Association between Mediterranean diet adherence and dyslipidaemia in a cohort of adults living in the Mediterranean area. Int. J. Food Sci. Nutr. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zappala, G.; Buscemi, S.; Mule, S.; La Verde, M.; D’Urso, M.; Corleo, D.; Marranzano, M. High adherence to Mediterranean diet, but not individual foods or nutrients, is associated with lower likelihood of being obese in a Mediterranean cohort. In Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity; Springer: Berlin, Germany, 2017; pp. 1–10. [Google Scholar]
- Bobermin, L.D.; Wartchow, K.M.; Flores, M.P.; Leite, M.C.; Quincozes-Santos, A.; Gonçalves, C.A. Ammonia-induced oxidative damage in neurons is prevented by resveratrol and lipoic acid with participation of heme oxygenase 1. Neurotoxicology 2015, 49, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Verkhratsky, A.; Parpura, V. Pathological role for exocytotic glutamate release from astrocytes in hepatic encephalopathy. Curr. Neuropharmacol. 2014, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Görg, B.; Qvartskhava, N.; Bidmon, H.-J.; Palomero-Gallagher, N.; Kircheis, G.; Zilles, K.; Häussinger, D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology 2010, 52, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Karababa, A.; Görg, B.; Schliess, F.; Häussinger, D. O-GlcNAcylation as a novel ammonia-induced posttranslational protein modification in cultured rat astrocytes. Metab. Brain Dis. 2014, 29, 975–982. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.M.V.; Piñeiro, C.C.; Leite, M.C.; Brolese, G.; Tramontina, F.; Feoli, A.M.; Gottfried, C.; Gonçalves, C.A. Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell. Mol. Neurobiol. 2007, 27, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Gottfried, C. Resveratrol modulates astroglial functions: Neuroprotective hypothesis. Ann. N Y. Acad. Sci. 2011, 1215, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V. Hepatic encephalopathy: Effects of liver failure on brain function. Nat. Rev. Neurosci. 2013, 14, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Vacante, M.; Drago, F.; Bertino, G.; Motta, M.; Giordano, M.; et al. Endozepine-4 levels are increased in hepatic coma. World J. Gastroenterol. 2015, 21, 9103–9110. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Thangthaeng, N.; Miller, M.G.; Shukitt-Hale, B. Effects of pterostilbene and resveratrol on brain and behavior. Neurochem. Int. 2015, 89, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem. Biophys. Res. Commun. 2006, 344, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; D’Amico, F.; Mazzarino, M.C.; Malaguarnera, L. Modulation of chitotriosidase during macrophage differentiation. Cell Biochem. Biophys. 2013, 66, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Giordano, M.; Motta, M.; Bertino, G.; Pennisi, M.; Neri, S.; Malaguarnera, M.; Li Volti, G.; Galvano, F. l-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J. Gastroenterol. 2011, 17, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- La Greca, G.; Santangelo, A.; Primo, S.; Sofia, M.; Latteri, S.; Russello, D.; Magro, G. Clinical and diagnostic problems of desmoid-type fibromatosis of the mesentery: Case report and review of the literature. Ann. Ital. Chir. 2014, 85. [Google Scholar]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFκB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Ferrando, S.J.; Javitch, J.A. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am. J. Psychiatry 2010, 167, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Wu, J.; Xu, H.; Li, G.; Zhu, D.; Yue, Q.; Liu, H.; Zhang, Y.; Sun, D.; et al. Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by β-amyloid through activation of SIRT1/Akt1 pathway. Biofactors 2014, 40, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, D.R.; Tranah, E.J.; Shawcross, D.L. Pathogenesis of hepatic encephalopathy: Role of ammonia and systemic inflammation. J. Clin. Exp. Hepatol. 2015, 5, S7–S20. [Google Scholar] [CrossRef] [PubMed]
- Finnell, J.E.; Lombard, C.M.; Melson, M.N.; Singh, N.P.; Nagarkatti, M.; Nagarkatti, P.; Fadel, J.R.; Wood, C.S.; Wood, S.K. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain Behav. Immun. 2017, 59, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Latteri, S.; Sofia, M.; Ricotta, A.; Castello, G.; Chisari, A.; Randazzo, V.; La Greca, G. Appendicular tuberculosis: The resurgence of an old disease with difficult diagnosis. World J. Gastroenterol. 2010, 16, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.-W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Olde Damink, S.W.M.; Hayes, P.C.; Deutz, N.E.P.; Lee, A. Pathogenesis of intracranial hypertension in acute liver failure: Inflammation, ammonia and cerebral blood flow. J. Hepatol. 2004, 41, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bosoi, C.R.; Rose, C.F. Oxidative stress: A systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rama Rao, K.V.; Norenberg, M.D. Brain energy metabolism and mitochondrial dysfunction in acute and chronic hepatic encephalopathy. Neurochem. Int. 2012, 60, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zwingmann, C. The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab. Brain Dis. 2007, 22, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Yurdaydin, C.; Hörtnagl, H.; Steindl, P.; Zimmermann, C.; Pifl, C.; Singer, E.A.; Roth, E.; Ferenci, P. Increased serotoninergic and noradrenergic activity in hepatic encephalopathy in rats with thioacetamide-induced acute liver failure. Hepatology 1990, 12, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lozeva, V.; Tuomisto, L.; Sola, D.; Plumed, C.; Hippeläinen, M.; Butterworth, R. Increased density of brain histamine H(1) receptors in rats with portacaval anastomosis and in cirrhotic patients with chronic hepatic encephalopathy. Hepatology 2001, 33, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Steindl, P.E.; Finn, B.; Bendok, B.; Rothke, S.; Zee, P.C.; Blei, A.T. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann. Intern. Med. 1995, 123, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.F.; Jones, E.A. Hepatic encephalopathy and the gamma-aminobutyric-acid neurotransmitter system. Lancet 1982, 319, 18–20. [Google Scholar] [CrossRef]
- Ahboucha, S.; Pomier-Layrargues, G.; Butterworth, R.F. Increased brain concentrations of endogenous (non-benzodiazepine) GABA-A receptor ligands in human hepatic encephalopathy. Metab. Brain Dis. 2004, 19, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Als-Nielsen, B.; Gluud, L.L.; Gluud, C. Dopaminergic agonists for hepatic encephalopathy. Cochrane Database Syst. Rev. 2004, 18. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Chenet, A.L.; Duarte, A.R.; Scaini, G.; Quevedo, J. Molecular Mechanisms Underlying the Anti-depressant Effects of Resveratrol: A Review. Mol. Neurobiol. 2017, 55, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; You, W.; Zhang, X.; Li, S.; Barish, P.A.; Vernon, M.M.; Du, X.; Li, G.; Pan, J.; et al. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. 2010, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, C.; Wang, C.; Zhang, J.-F.; Zhou, W.H.; Cui, W.-G.; Ye, F.; Xu, Y. Chronic resveratrol treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with mononeuropathy: Involvement of serotonergic system. Neuropharmacology 2014, 85, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M. Acetyl-l-carnitine in hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Bertino, G.; Chisari, G.; Motta, M.; Vecchio, M.; Vacante, M.; Caraci, F.; Greco, C.; Drago, F.; Nunnari, G.; et al. Silybin supplementation during HCV therapy with pegylated interferon-α plus ribavirin reduces depression and anxiety and increases work ability. BMC Psychiatry 2016, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Bella, R.; Vacante, M.; Giordano, M.; Malaguarnera, G.; Gargante, M.P.; Motta, M.; Mistretta, A.; Rampello, L.; Pennisi, G. Acetyl-L-carnitine reduces depression and improves quality of life in patients with minimal hepatic encephalopathy. Scand. J. Gastroenterol. 2011, 46, 750–759. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Resveratrol Group 35 pt | Placebo Group 35 pt | p |
|---|---|---|---|
| Male/Female | 25/10 | 23/12 | N.S |
| Age (range) | 39–60 | 35–60 | N.S |
| SBP (mmHg) | 144.00 ± 18.20 | 145.00 ± 19.10 | N.S |
| DBP (mmHg) | 82.10 ± 10.40 | 81.80 ± 10.50 | N.S |
| Heart Rate (bpm) | 77 ± 8 | 75 ± 9 | N.S |
| BMI (kg/m2) | 25.80 ± 2.40 | 26.10 ± 2.70 | N.S |
| Smokers/No Smokers | 15/20 | 14/21 | N.S |
| Cirrhosis etiology | |||
| Post Hepatitis B | 10 | 11 | N.S |
| Post Hepatitis C | 16 | 14 | N.S |
| Alcoholism | 3 | 2 | N.S |
| Unknown | 6 | 8 | N.S |
| Child-Pugh Class | |||
| Grade A | 20 | 19 | N.S |
| Grade B | 15 | 16 | N.S |
| Characteristics | Resveratrol Group 35 | Placebo Group 35 | After RV→Placebo | ||||
|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | p | |
| Urea | 51.80 ± 6.10 | 48.20 ± 6.40 | 0.019 | 51.40 ± 5.90 | 50.20 ± 6.10 | N.S | N.S |
| Ammonia | 66.20 ± 7.80 | 41.40 ± 6.80 | <0.001 | 62.80 ± 7.40 | 59.70 ± 6.70 | N.S | <0.001 |
| AST | 87.80 ± 10.70 | 40.40 ± 9.80 | <0.001 | 80.40 ± 9.80 | 75.20 ± 8.70 | 0.022 | <0.001 |
| ALT | 96.40 ± 11.80 | 78.40 ± 8.70 | <0.001 | 91.70 ± 10.20 | 84.20 ± 10.20 | 0.003 | 0.013 |
| γ-GT | 46.10 ± 6.70 | 40.40 ± 6.40 | <0.001 | 40.80 ± 6.40 | 40.50 ± 6.10 | N.S | N.S |
| Prothrombine time | 15.40 ± 1.80 | 15.00 ± 1.20 | N.S | 15.50 ± 1.70 | 15.10 ± 1.60 | N.S | N.S |
| Bilirubin | 2.00 ± 0.40 | 2.10 ± 0.50 | N.S | 2.10 ± 0.40 | 2.00 ± 0.50 | N.S | N.S |
| Albumin | 3.50 ± 0.50 | 3.70 ± 0.80 | N.S | 3.60 ± 0.50 | 3.60 ± 0.70 | N.S | N.S |
| Characteristics | Resveratrol Group | p | Placebo Group | p | After treatment RV vs. Placebo | ||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | p | |||
| Physical function | 60.20 ± 7.90 | 72.40 ± 8.10 | <0.001 | 60.10 ± 7.00 | 61.80 ± 7.20 | N.S | <0.001 |
| Role physical | 67.40 ± 9.10 | 71.80 ± 8.40 | N.S | 64.20 ± 8.90 | 67.10 ± 8.70 | N.S | 0.025 |
| Body pain | 60.20 ± 7.80 | 66.40 ± 7.40 | 0.001 | 60.80 ± 7.90 | 62.40 ± 7.50 | N.S | 0.028 |
| General health | 55.10 ± 7.20 | 65.80 ± 6.80 | <0.001 | 57.20 ± 7.30 | 59.20 ± 7.50 | N.S | <0.001 |
| Vitality | 59.20 ± 6.20 | 64.00 ± 6.40 | 0.002 | 61.80 ± 6.50 | 60.70 ± 6.70 | N.S | 0.039 |
| Role emotional | 61.70 ± 7.70 | 65.20 ± 7.40 | N.S | 61.90 ± 7.40 | 62.80 ± 7.20 | N.S | N.S |
| Mental health | 58.70 ± 8.70 | 64.80 ± 7.20 | 0.002 | 62.40 ± 7.70 | 62.40 ± 7.40 | N.S | N.S |
| Social function | 60.20 ± 7.10 | 69.80 ± 7.50 | <0.001 | 61.40 ± 7.50 | 62.70 ± 7.70 | N.S | <0.001 |
| Beck Depression Inventory | 28.10 ± 3.40 | 20.40 ± 3.20 | <0.001 | 27.80 ± 3.50 | 26.40 ± 3.20 | N.S | <0.001 |
| STAI | 45.40 ± 7.90 | 37.80 ± 6.10 | <0.001 | 44.90 ± 7.40 | 43.80 ± 7.20 | N.S | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaguarnera, G.; Pennisi, M.; Bertino, G.; Motta, M.; Borzì, A.M.; Vicari, E.; Bella, R.; Drago, F.; Malaguarnera, M. Resveratrol in Patients with Minimal Hepatic Encephalopathy. Nutrients 2018, 10, 329. https://doi.org/10.3390/nu10030329
Malaguarnera G, Pennisi M, Bertino G, Motta M, Borzì AM, Vicari E, Bella R, Drago F, Malaguarnera M. Resveratrol in Patients with Minimal Hepatic Encephalopathy. Nutrients. 2018; 10(3):329. https://doi.org/10.3390/nu10030329
Chicago/Turabian StyleMalaguarnera, Giulia, Manuela Pennisi, Gaetano Bertino, Massimo Motta, Antonio Maria Borzì, Enzo Vicari, Rita Bella, Filippo Drago, and Michele Malaguarnera. 2018. "Resveratrol in Patients with Minimal Hepatic Encephalopathy" Nutrients 10, no. 3: 329. https://doi.org/10.3390/nu10030329





