Self-Reported Fractures in Dermatitis Herpetiformis Compared to Coeliac Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Controls, and Study Design
2.2. Questionnaires
2.3. Fractures
2.4. Statistical Analysis
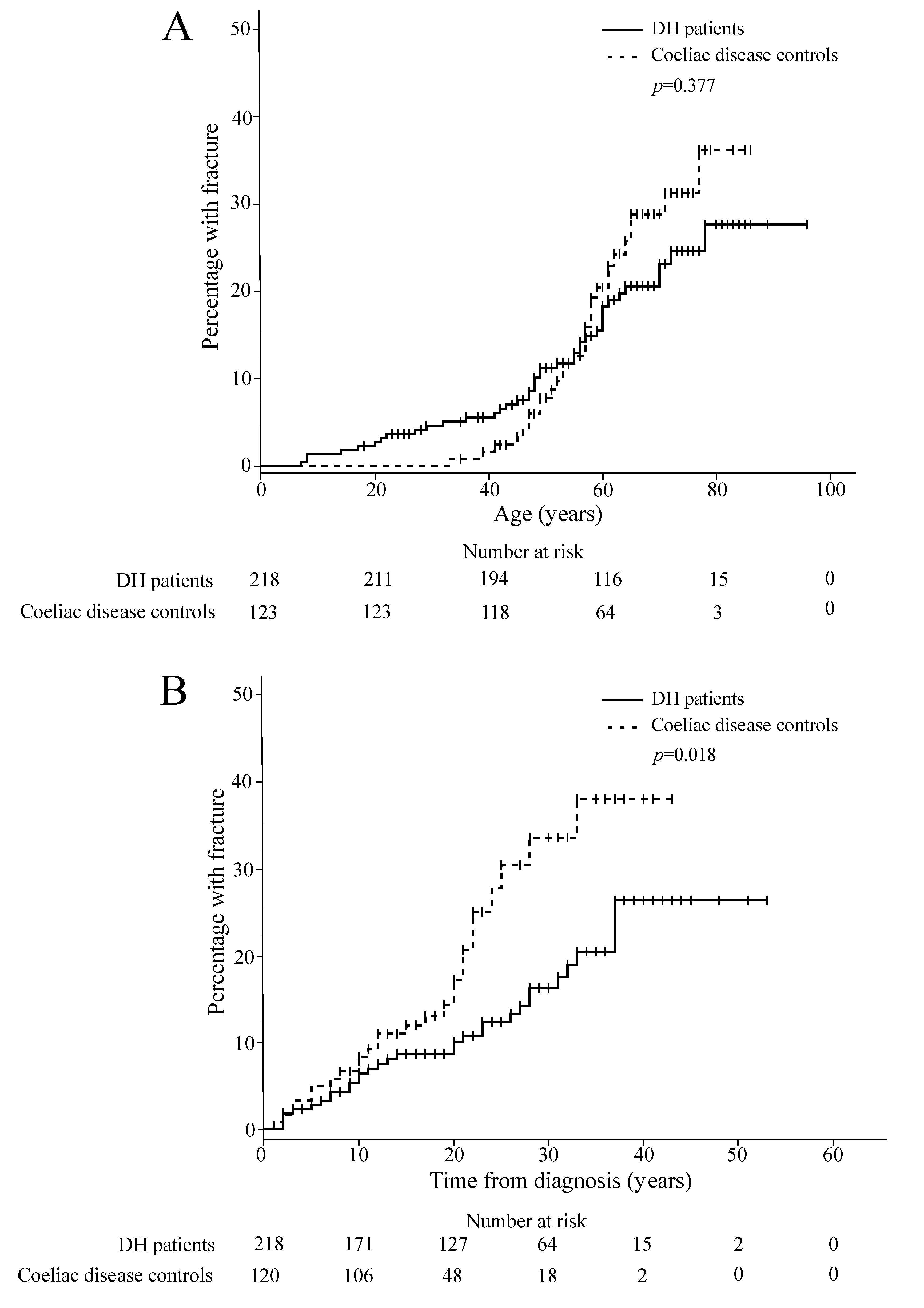
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| DH Patients (n = 222) | Coeliac Disease Controls (n = 129) | |||||
|---|---|---|---|---|---|---|
| With Fracture (n = 45) | Without Fracture (n = 177) | p-Value | With Fracture (n = 35) | Without Fracture (n = 94) | p-Value | |
| GSRS | ||||||
| Total | 1.9 (1.3–2.3) | 1.6 (1.3–2.1) | 0.191 | 1.9 (1.5–2.5) | 1.7 (1.4–2.5) | 0.472 |
| Diarrhoea | 1.7 (1.0–2.3) | 1.3 (1.0–2.0) | 0.116 | 2.0 (1.0–2.7) | 1.7 (1.0–2.5) | 0.635 |
| Indigestion | 2.0 (1.3–2.5) * | 1.8 (1.5–2.5) | 0.630 | 2.4 (1.8–2.8) | 2.0 (1.5–3.0) | 0.343 |
| Constipation | 1.3 (1.0–2.3) | 1.3 (1.0–2.3) | 0.568 | 1.7 (1.3–2.5) | 1.7 (1.0–2.7) | 0.572 |
| Pain | 1.7 (1.2–2.3) | 1.3 (1.0–2.0) | 0.130 | 1.7 (1.3–2.3) | 1.7 (1.3–2.3) | 0.517 |
| Reflux | 1.5 (1.0–2.0) | 1.0 (1.0–1.5) | 0.012 | 1.5 (1.0–2.5) | 1.0 (1.0–2.0) | 0.083 |
| PGWB | ||||||
| Total | 106 (94–113) | 112 (101–119) | 0.006 | 110 (93–120) | 106 (97–116) | 0.629 |
| Anxiety | 25 (22–27) | 26 (23–28) | 0.020 | 27 (21–29) | 25 (23–28) | 0.757 |
| Depression | 17 (16–18) | 18 (16–18) | 0.311 | 17 (15–18) | 17 (15–18) | 0.948 |
| Well-being | 17 (15–19) | 18 (16–20) | 0.007 | 18 (15–19) | 17 (16–20) | 0.941 |
| Self control | 16 (15–17) | 16 (15–17) | 0.052 | 16 (13–17) | 16 (14–17) | 0.888 |
| General health | 13 (11–15) | 15 (13–16 | 0.012 | 13 (11–16) | 13 (11–15) | 0.712 |
| Vitality | 18 (17–21) | 20 (17–21) | 0.029 | 19 (17–20) | 18 (16–20) | 0.665 |
References
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; Van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and Celiac Disease. Curr. Osteoporos Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Mengoli, C.; Bergonzi, M.; Corazza, G.R. Bone mass and mineral metabolism alterations in adult celiac disease: Pathophysiology and clinical approach. Nutrients 2013, 5, 4786–4799. [Google Scholar] [CrossRef] [PubMed]
- Mazure, R.; Vazquez, H.; Gonzalez, D.; Mautalen, C.; Pedreira, S.; Boerr, L.; Bai, J.C. Bone mineral affection in asymptomatic adult patients with celiac disease. Am. J. Gastroenterol. 1994, 89, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Mustalahti, K.; Collin, P.; Sievänen, H.; Salmi, J.; Mäki, M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet 1999, 354, 744–745. [Google Scholar] [CrossRef]
- Corazza, G.R.; Di Sario, A.; Cecchetti, L.; Tarozzi, C.; Corrao, G.; Bernardi, M.; Gasbarrini, G. Bone mass and metabolism in patients with celiac disease. Gastroenterology 1995, 109, 122–128. [Google Scholar] [CrossRef]
- Szymczak, J.; Bohdanowicz-Pawlak, A.; Waszczuk, E.; Jakubowska, J. Low bone mineral density in adult patients with coeliac disease. Endokrynol. Pol. 2012, 63, 270–276. [Google Scholar] [PubMed]
- Heikkilä, K.; Pearce, J.; Mäki, M.; Kaukinen, K. Celiac disease and bone fractures: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Reunala, T. Dermatitis herpetiformis: A cutaneous manifestation of coeliac disease. Ann. Med. 2016, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.; Riches, D.J.; Seah, P.P.; Hoffbrand, A.V. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet 1973, 301, 288–291. [Google Scholar] [CrossRef]
- Hervonen, K.; Alakoski, A.; Salmi, T.T.; Helakorpi, S.; Kautiainen, H.; Kaukinen, K.; Pukkala, E.; Collin, P.; Reunala, T. Reduced mortality in dermatitis herpetiformis: A population-based study of 476 patients. Br. J. Dermatol. 2012, 167, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Alakoski, A.; Salmi, T.T.; Hervonen, K.; Kautiainen, H.; Salo, M.; Kaukinen, K.; Reunala, T.; Collin, P. Chronic gastritis in dermatitis herpetiformis: A controlled study. Clin. Dev. Immunol. 2012, 2012, 640630. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.R.; Logan, R.F.A.; Hubbard, R.B.; West, J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: A cohort study. Aliment. Pharmacol. Ther. 2008, 27, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Jorizzo, R.A.; Veneto, G.; Cecchetti, L.; Gasbarrini, G.; Corazza, G.R. Bone mass and metabolism in dermatitis herpetiformis. Dig. Dis. Sci. 1999, 44, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Lorinczy, K.; Juhász, M.; Csontos, Á.; Fekete, B.; Terjék, O.; Lakatos, P.L.; Miheller, P.; Kocsis, D.; Kárpáti, S.; Tulassay, Z.; et al. Does dermatitis herpetiformis result in bone loss as coeliac disease does ? A cross sectional study. Rev. Esp. Enferm. Dig. 2013, 105, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Abuzakouk, M.; Barnes, L.; O’Gorman, N.; O’Grady, A.; Mohamed, B.; McKenna, M.J.; Freaney, R.; Feighery, C. Dermatitis herpetiformis: No evidence of bone disease despite evidence of enteropathy. Dig. Dis. Sci. 2007, 52, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Lheure, C.; Ingen-Housz-Oro, S.; Guignard, S.; Inaoui, R.; Jolivet, B.; Chevalier, X.; Wolkenstein, P.; Fautrel, B.; Chosidow, O. Dermatitis herpetiformis and bone mineral density: Analysis of a French cohort of 53 patients. Eur. J. Dermatol. 2017, 27, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zone, J.J.; Meyer, L.J.; Petersen, M.J. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch. Dermatol. 1996, 132, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Dimenäs, E.; Carlsson, G.; Glise, H.; Israelsson, B.; Wiklund, I. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand. J. Gastroenterol. Suppl. 1996, 221, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Kärner, A.; Hallert, C. Psychological well-being of adult coeliac patients treated for 10 years. Dig. Liver Dis. 2006, 38, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjodin, I.; Dotevall, G. GSRS-A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Suraci, E.; Nazionale, I.; Abenavoli, L.; Imeneo, M.; Luzza, F. Bone mineralization in celiac disease. Gastroenterol. Res. Pract. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ilus, T.; Lähdeaho, M.-L.; Salmi, T.; Haimila, K.; Partanen, J.; Saavalainen, P.; Huhtala, H.; Mäki, M.; Collin, P.; Kaukinen, K. Persistent duodenal intraepithelial lymphocytosis despite a long-term strict gluten-free diet in celiac disease. Am. J. Gastroenterol. 2012, 107, 1563–1569. [Google Scholar] [CrossRef]
- Kemppainen, T.; Kroger, H.; Janatuinen, E.; Arnala, I.; Kosma, V.M.; Pikkarainen, P.; Julkunen, R.; Jurvelin, J.; Alhava, E.; Uusitupa, M. Osteoporosis in adult patients with celiac disease. Bone 1999, 24, 249–255. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, Y.; Li, H.; Sun, W.; Liu, J. Proton-pump inhibitors and risk of fractures: An update meta-analysis. Osteoporos. Int. 2016, 27, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, K.; Honkanen, R.; Heikkinen, L.; Kröger, H.; Saarikoski, S. Validity of self-reports of fractures in perimenopausal women. Am. J. Epidemiol. 1999, 150, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mock, C.; Acheampong, F.; Adjei, S.; Koepsell, T. The effect of recall on estimation of incidence rates for injury in Ghana. Int. J. Epidemiol. 1999, 28, 750–755. [Google Scholar] [CrossRef] [PubMed]
| DH Patients (n = 222) | Coeliac Disease Controls (n = 129) | p-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Female | 101 | 45 | 104 | 81 | <0.001 |
| Age at diagnosis, median (range), years | 37 (5–78) | 42 (7–72) | 0.027 | ||
| Age at the time of the study, median (range), years | 65 (18–96) | 66 (35–86) | 0.654 | ||
| BMI 1 at the time of the study, median (range), kg/m2 | 26 (17–40) | 26 (15–46) | 0.714 | ||
| Reported fractures | 45 | 20 | 35 | 27 | 0.140 |
| Before diagnosis | 13 | 6 | 3 | 3 | 0.143 |
| After diagnosis | 31 | 14 | 26 | 22 | 0.080 |
| Reported multiple fractures | 15 | 7 | 12 | 9 | 0.388 |
| DH Patients (n = 222) | p-Value | Coeliac Disease Controls (n = 129) | p-Value | |||
|---|---|---|---|---|---|---|
| With Fracture (n = 45) | Without Fracture (n = 177) | With Fracture (n = 35) | Without Fracture (n = 94) | |||
| Female, % | 58 * | 42 | 0.064 | 97 | 74 | 0.002 |
| Age at diagnosis, median (range), years | 34 (7–78) * | 37 (5–78) | 0.652 | 45 (23–59) | 40 (7–72) | 0.428 |
| Age at the time of the study, median (range), years | 68 (22–85) | 65 (18–96) | 0.343 | 68 (51–82) | 63 (35–86) | 0.020 |
| Smoking at the time of the study, % | 0.581 | 0.218 | ||||
| Non-smoker | 69 | 68 | 66 | 70 | ||
| Ex-smoker | 17 | 22 | 31 | 20 | ||
| Current smoker | 14 | 10 | 3 | 10 | ||
| Exercise at the time of the study, % | 0.341 | 0.396 | ||||
| Not at all | 11 | 11 | 6 | 10 | ||
| 1 to 3 times per week | 55 | 43 | 57 | 44 | ||
| 4 to 7 times per week | 34 | 46 | 37 | 46 | ||
| Dietary adherence to GFD at the time of the study, % | 0.858 | 0.646 | ||||
| Strict 1 | 70 | 73 | 80 | 86 | ||
| Dietary lapses less than once a month | 21 | 18 | 17 | 10 | ||
| Dietary lapses more than once a month | 7 | 8 | 3 | 3 | ||
| Normal diet | 2 | 1 | 0 | 1 | ||
| Diagnosed with osteoporosis, % | 11 * | 2 | 0.024 | 40 | 11 | 0.001 |
| Multiple long-term illnesses 2, % | 33 | 19 | 0.033 | 43 | 26 | 0.057 |
| Use of long-term medication at the time of the study, % | ||||||
| Proton-pump inhibitor | 21 | 9 | 0.029 | 26 | 11 | 0.034 |
| Hormone replacement therapy | 14 | 4 | 0.008 | 3 | 11 | 0.155 |
| Any glucocorticoid medication | 18 | 12 | 0.349 | 26 | 16 | 0.205 |
| Vitamin D and calcium supplementation | 39 | 10 | <0.001 | 43 | 25 | 0.045 |
| Bisphosphonates | 5 | 3 | 0.545 | 9 | 6 | 0.506 |
| Diuretics | 21 | 9 | 0.028 | 11 | 8 | 0.491 |
| DH Patients | p-Value | ||
|---|---|---|---|
| With Fracture (n = 45) | Without Fracture (n = 177) | ||
| Year of DH diagnosis, median (IQR) | 1990 (1976–2000) | 1991 (1982–2002) | 0.076 |
| Duration of skin symptoms prior to DH diagnosis, median (IQR), months | 12 (6–60) | 10 (5–24) | 0.183 |
| Severity of skin symptom at diagnosis, % | 0.818 | ||
| Mild | 19 | 15 | |
| Moderate | 46 | 50 | |
| Severe | 35 | 35 | |
| Presence of gastrointestinal symptoms at the time of diagnosis, % | 47 | 49 | 0.886 |
| Small-bowel histology at diagnosis, % | 0.405 | ||
| Normal | 16 | 24 | |
| PVA 1 | 35 | 39 | |
| SVA/TVA 2 | 49 | 37 | |
| Use of dapsone after diagnosis, % | 79 | 77 | 0.854 |
| Duration of dapsone, median (IQR), months | 60 (12–171) | 24 (12–60) | 0.031 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasternack, C.; Mansikka, E.; Kaukinen, K.; Hervonen, K.; Reunala, T.; Collin, P.; Huhtala, H.; Mattila, V.M.; Salmi, T. Self-Reported Fractures in Dermatitis Herpetiformis Compared to Coeliac Disease. Nutrients 2018, 10, 351. https://doi.org/10.3390/nu10030351
Pasternack C, Mansikka E, Kaukinen K, Hervonen K, Reunala T, Collin P, Huhtala H, Mattila VM, Salmi T. Self-Reported Fractures in Dermatitis Herpetiformis Compared to Coeliac Disease. Nutrients. 2018; 10(3):351. https://doi.org/10.3390/nu10030351
Chicago/Turabian StylePasternack, Camilla, Eriika Mansikka, Katri Kaukinen, Kaisa Hervonen, Timo Reunala, Pekka Collin, Heini Huhtala, Ville M. Mattila, and Teea Salmi. 2018. "Self-Reported Fractures in Dermatitis Herpetiformis Compared to Coeliac Disease" Nutrients 10, no. 3: 351. https://doi.org/10.3390/nu10030351





