Effects of Low Versus Moderate Glycemic Index Diets on Aerobic Capacity in Endurance Runners: Three-Week Randomized Controlled Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Protocol
2.2.1. Study Visits
2.2.2. Study Diets
2.2.3. Anthropometry and Body Composition
2.2.4. Daily Energy Expenditure
2.2.5. Exercise Tests
Aerobic Capacity (ICT Test)
Endurance Performance
2.2.6. Statistical Analysis
3. Results
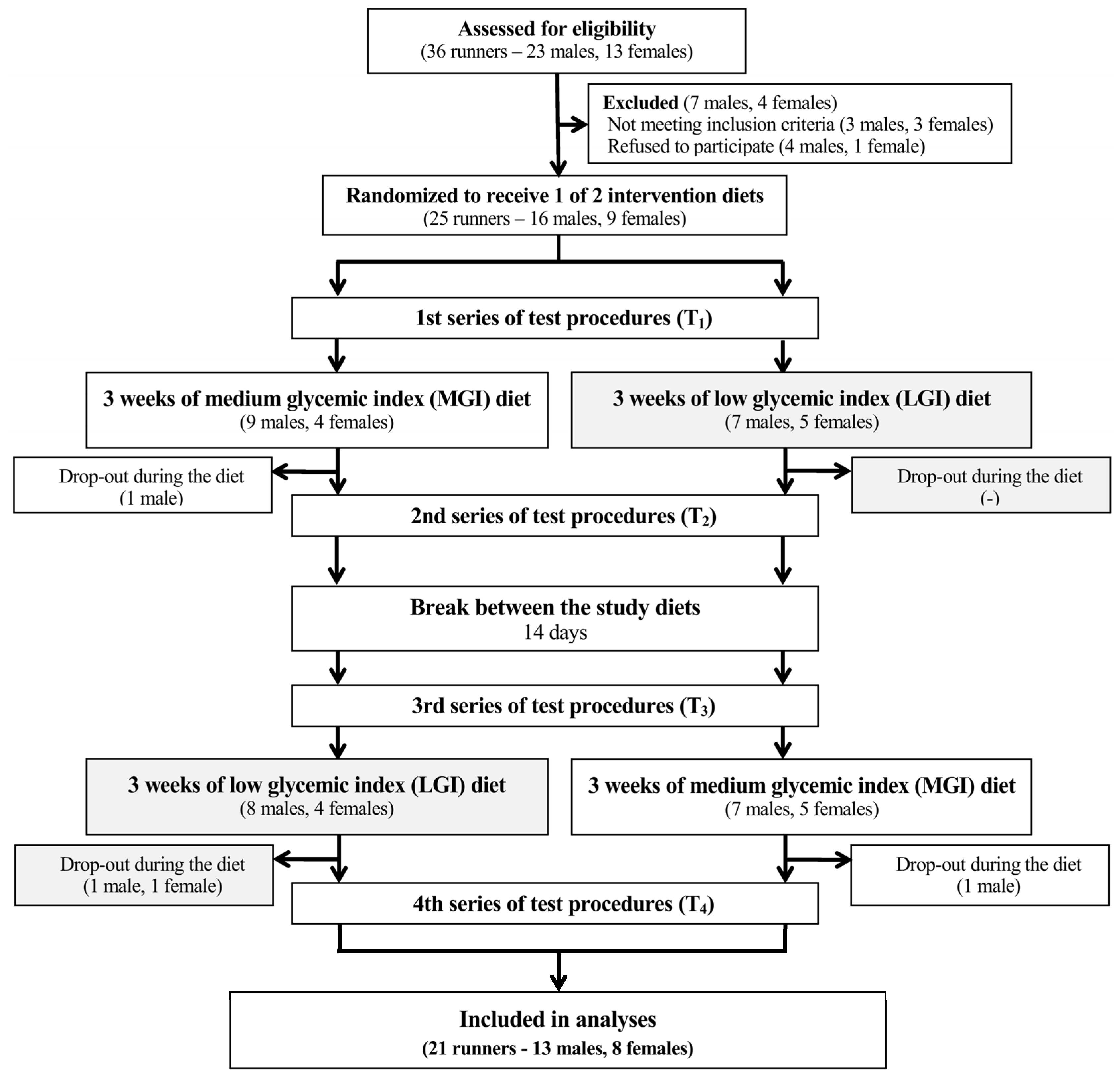
3.1. Participants and Adherence
3.2. Body Mass and Composition
3.3. Aerobic Capacity
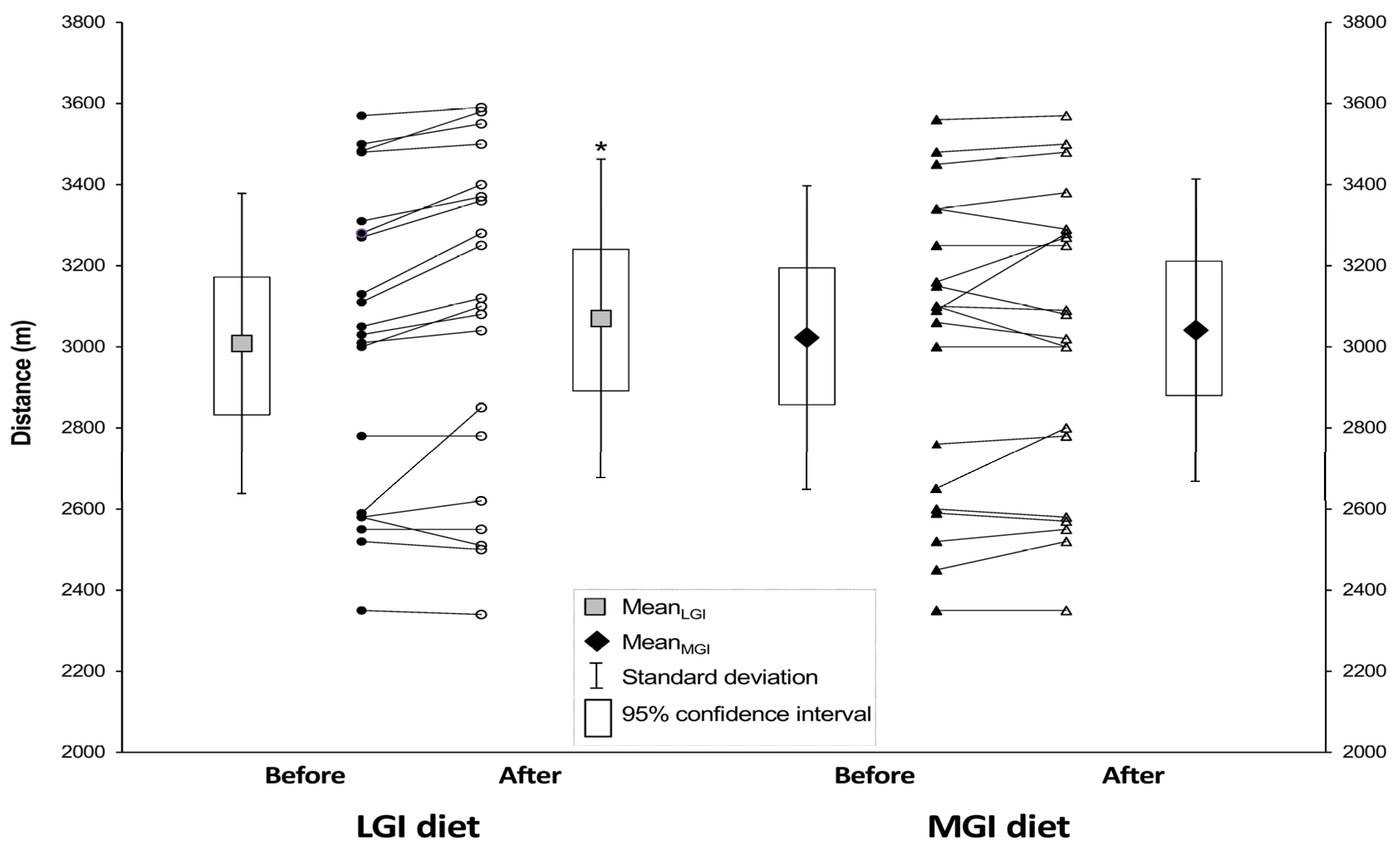
3.4. Endurance Performance
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Availability of Data and Material
Conflicts of Interest
References
- Burke, L.M.; Kiens, B.; Ivy, J.L. Carbohydrates and fat for training and recovery. J. Sports Sci. 2004, 22, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Aston, L.M.; Gambell, J.M.; Lee, D.M.; Bryant, S.P.; Jebb, S.A. Determination of the glycaemic index of various staple carbohydrate-rich foods in the UK diet. Eur. J. Clin. Nutr. 2008, 62, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Lightowler, H.J.; Strik, C.M.; Storey, M. Glycaemic index values for commercially available potatoes in Great Britain. Br. J. Nutr. 2005, 94, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Tofas, T.; Fatouros, I.; Nikolaidis, M.G.; Paschalis, V.; Yfanti, C.; Raptis, S.; Koutedakis, Y. The effects of low and high glycemic index foods on exercise performance and beta-endorphin responses. J. Int. Soc. Sports Nutr. 2011, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Heslin, C.J.; Rezende, R.S. Metabolic and performance effects of raisins versus sports gel as pre-exercise feedings in cyclists. J. Strength Cond. Res. 2007, 21, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; Midgley, A.W.; Thomas, G.; Thurlow, S.; McNaughton, L.R. The effect of low- and high- glycemic index meals on time trial performance. Int. J. Sports Physiol. Perform. 2009, 4, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; Midgley, A.W.; Thomas, G.; Thurlow, S.; McNaughton, L.R. Effect of the glycaemic index of a pre-exercise meal on metabolism and cycling time trial performance. J. Sci. Med. Sport 2010, 13, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.E.; Elliott, E.J.; Baur, L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst. Rev. 2007, 3, CD005105. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Siu, P.M.; Lok, A.; Chen, Y.J.; Morris, J.; Lam, C.W. Effect of the glycaemic index of pre-exercise carbohydrate meals on running performance. Eur. J. Sport Sci. 2008, 8, 23–33. [Google Scholar] [CrossRef]
- Wu, C.L.; Williams, C. A low glycemic index meal before exercise improves endurance running capacity in men. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Keenan, J.; Angus, D.J.; Campbell, S.E.; Garnham, A.P. Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: Effect of the glycemic index. J. Appl. Physiol. 2000, 89, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Burdon, C.A.; Spronk, I.; Cheng, H.L.; O’Connor, H.T. Effect of glycemic index of a pre-exercise meal on endurance exercise performance: A systematic review and meta-analysis. Sports Med. 2017, 47, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Heung-Sang Wong, S.; Sun, F.H.; Chen, Y.J.; Li, C.; Zhang, Y.J.; Ya-Jun Huang, W. Effect of pre-exercise carbohydrate diets with high vs. low glycemic index on exercise performance: A meta-analysis. Nutr. Rev. 2017, 75, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wong, S.H.; Xu, X.; Hao, X.; Wong, C.K.; Lam, C.W. Effect of CHO loading patterns on running performance. Int. J. Sports Med. 2008, 29, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, S.; Higgins, S.; Abraham, T.; Taylor, P.; Vizbaraite, D.; Malkova, D. The effect of glycaemic index of high carbohydrate diets consumed over 5 days on exercise energy metabolism and running capacity in males. J. Sports Sci. 2009, 27, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Stevenson, E.J. New perspectives on nutritional interventions to augment lipid utilisation during exercise. Br. J. Nutr. 2012, 107, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Woźniewicz, M.; Bajerska, J.; Jeszka, J. Comparison of accuracy of various non-calorimetric methods measuring energy expenditure at different intensities. Hum. Mov. 2013, 14, 161–167. [Google Scholar] [CrossRef]
- Clapp, J.F.; Lopez, B. Low-versus high-glycemic index diets in women: Effects on caloric requirement, substrate utilization and insulin sensitivity. Metab. Syndr. Relat. Disord. 2007, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.; Miller, E.R.; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The OmniCarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, S.H.; Williams, C.; Stevenson, E.; Nute, M. Effects of the glycemic index of breakfast on metabolic responses to brisk walking in females. Eur. J. Clin. Nutr. 2007, 61, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.H.; O’Reilly, J.; Li, L.; Wong, S.H. Effect of the glycemic index of pre-exercise snack bars on substrate utilization during subsequent exercise. Int. J. Food Sci. Nutr. 2013, 64, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research and recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef]
- Bentzur, K.M.; Kravitz, L.; Lockner, D.W. Evaluation of the BOD POD for estimating percent body fat in collegiate track and field female athletes: A comparison of four methods. J. Strength Cond. Res. 2008, 22, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Robson, P.J.; Totton, M. Energy expenditure by heart rate in children: An evaluation of calibration techniques. Med. Sci. Sports Exerc. 2000, 32, 1513–1539. [Google Scholar] [CrossRef] [PubMed]
- Howley, E.T.; Bassett, D.R., Jr.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.M.; Jones, A.M.; Davison, R.C.R.; Bromley, P.D.; Mercer, T. Sport Testing. Sport and Exercise Physiology Testing Guidelines: The British Association of Sport and Exercise Sciences; Routledge—Taylor and Francis Group: Abingdon, UK, 2007; Volume 2, pp. 112–119. [Google Scholar]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting the anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.T.; Thewlis, D.; Tsiros, M.D.; Brown, N.A.T.; Buckley, J.D. Six-week transition to minimalist shoes improves running economy and time-trial performance. J. Sci. Med. Sport 2017. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A. Validity of Cooper’s 12-minute run test for estimation of maximum oxygen uptake in male university students. Biol. Sport 2015, 32, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Garatachea, N.; Almeida, R.; Casajús, J.A.; Bailón, R. Validation of heart rate monitor Polar RS800 for heart rate variability analysis during exercise. J. Strength Cond. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wong, S.H.; Wong, C.K.; Lam, C.W.; Huang, Y.J.; Siu, P.M. Effect of preexercise meals with different glycemic indices and loads on metabolic responses and endurance running. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; Midgley, A.; Vince, R.; McNaughton, L.R. The effects of low and high glycemic index 24-h recovery diets on cycling time trial performance. J. Sports Med. Phys. Fit. 2011, 51, 233–240. [Google Scholar]
- Karamanolis, I.A.; Laparidis, K.S.; Volaklis, K.A.; Douda, H.T.; Tokmakidis, S.P. The effects of pre-exercise glycemic index food on running capacity. Int. J. Sports Med. 2011, 32, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L.; Williams, C.; Tsintzas, K.; Boobis, L. Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise. J. Appl. Physiol. 2005, 99, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Levere, X.; Batandier, C.; Fontaine, E. Choosing the right substrate. Nov. Found Symp. 2007, 280, 108–121. [Google Scholar]
- Cermak, N.M.; van Loon, L.J. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Chilibeck, P.D.; Ciona, D.; Vandenberg, A.; Zello, G.A. The effects of low- and high-glycemic index foods on high-intensity intermittent exercise. Int. J. Sports Physiol. Perform. 2009, 4, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A. Is the ventilatory threshold coincident with maximal fat oxidation during submaximal exercise in women? J. Sports Med. Phys. Fit. 2000, 40, 209–216. [Google Scholar]
- Peric, R.; Meucci, M.; Nikolovski, Z. Fat utilization during high-intensity exercise: When does it end? Sports Med. Open 2016, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.E.; Mbambo, Z.; Kortekaas, E.; Wilson, G.; Lambert, M.I.; Noakes, T.D.; Dennis, S.C. Advantages of smaller body mass during distance running in warm, humid environments. Pflug. Arch. 2000, 441, 359–367. [Google Scholar] [CrossRef]
- O’Connor, H.; Slater, G. Losing, gaining and making weight for athletes. In Sport and Exercise Nutrition; Lanham-New, S., Stear, S., Sherriffs, S., Collins, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2011; pp. 210–232. [Google Scholar]
- Sedeaud, A.; Marc, A.; Marck, A.; Dor, F.; Schipman, J.; Dorsey, M.; Haida, A.; Berthelot, G.; Toussaint, J.F. BMI, a performance parameter for speed improvement. PLoS ONE. 2014, 9, e90183. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Jones, B. Alternatives to rapid weight loss in US wrestling. Int. J. Sports Med. 2010, 31, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, S.; Pipping Ekström, M.; Berg, C.M. The food and weight combat. A problematic fight for the elite combat sports athlete. Appetite 2012, 59, 234–242. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Baseline MGIbefore | MGI Diet | Baseline LGIbefore | LGI Diet |
|---|---|---|---|---|
| Energy intake (kcal) | 3060 ± 632 | 3190 ± 616 | 3062 ± 651 | 3174 ± 600 |
| Protein (g) | 141.2 ± 33.1 | 109.0 ± 20.8 * | 141.6 ± 33.8 | 109.7 ± 20.6 ‡ |
| Fat (g) | 102.8 ± 20.4 | 90.4 ± 16.2 * | 103.5 ± 21.2 | 90.4 ± 17.5 † |
| Carbohydrates (g) | 392.5 ± 83.4 | 485.2 ± 101.3 * | 389.8 ± 86.8 | 480.2 ± 96.8 ‡ |
| Dietary fiber (g) | 31.7 ± 2.6 | 38.1 ± 6.5 # | 31.9 ± 2.4 | 57.6 ± 8.0 ‡§ |
| Glycemic Index (GI) | 60 ± 3 | 63 ± 1 # | 60 ± 3 | 39 ± 1 ‡§ |
| Parameter | ANOVA | MGI Diet | LGI Diet | Carryover Effect | |
|---|---|---|---|---|---|
| p-Value | p-Value | ||||
| Body mass (kg) | Before | 0.0311 | 65.3 ± 11.3 | 65.7 ± 11.9 | 0.1153 |
| (60.2–70.5) | (60.2–71.1) | ||||
| After | 65.9 ± 12.0 | 65.1 ± 11.7 # | |||
| (60.4–71.3) | (59.7–70.4) | ||||
| Fat-free mass (kg) | Before | 0.5954 | 51.2 ± 8.4 | 51.3 ± 9.0 | 0.2541 |
| (47.4–55.0) | (47.3–55.4) | ||||
| After | 51.4 ± 8.4 | 51.2 ± 8.6 | |||
| (47.6–55.2) | (47.2–55.1) | ||||
| Fat mass (kg) | Before | 0.3828 | 14.1 ± 4.3 | 14.3 ± 4.7 | 0.3064 |
| (12.2–16.1) | (12.2–16.5) | ||||
| After | 14.5 ± 5.0 | 13.9 ± 4.5 | |||
| (12.2–16.8) | (11.8–16.0) | ||||
| Total body water (L) | Before | 0.2268 | 37.6 ± 6.2 | 38.0 ± 6.5 | 0.5317 |
| (34.8–40.4) | (35.0–41.0) | ||||
| After | 37.7 ± 6.2 | 37.8 ± 6.3 | |||
| (34.9–40.5) | (34.9–40.7) | ||||
| Fat-free mass (%) $ | Before | 0.8168 | 78.5 ± 4.3 | 78.4 ± 4.8 | 0.4444 |
| (76.6–80.5) | (76.2–80.6) | ||||
| After | 78.3 ± 4.8 | 78.9 ± 4.4 | |||
| (76.1–80.5) | (76.9–80.9) | ||||
| Fat mass (%) $ | Before | 0.8168 | 21.5 ± 4.3 | 21.6 ± 4.8 | 0.4444 |
| (19.5–23.4) | (19.4–23.8) | ||||
| After | 21.7 ± 4.8 | 21.1 ± 4.4 | |||
| (19.5–23.9) | (19.1–23.1) | ||||
| Total body water (%) $ | Before | 0.6500 | 57.7 ± 4.8 | 58.1 ± 4.3 | 0.6636 |
| (55.6–59.9) | (56.1–60.0) | ||||
| After | 57.6 ± 5.1 | 58.3 ± 4.0 | |||
| (55.3–59.9) | (56.5–60.1) |
| Parameter | ANOVA | MGI Diet | LGI Diet | Carryover Effect | |
|---|---|---|---|---|---|
| p-Value | p-Value | ||||
| O2max (L∙min−1) | Before | 0.0397 | 3.39 ± 0.86 | 3.45 ± 0.87 | 0.5905 |
| (3.00–3.78) | (3.05–3.85) | ||||
| After | 3.52 ± 0.84 * | 3.51 ± 0.82 | |||
| (3.14–3.90) | (3.14–3.88) | ||||
| O2max (mL∙min−1·kg−1) | Before | 0.1130 | 51.58 ± 9.43 | 52.26 ± 8.52 | 0.2673 |
| (47.29–55.87) | (48.38–56.14) | ||||
| After | 53.38 ± 8.76 | 53.90 ± 8.70 | |||
| (49.39–57.37) | (49.94–57.86) | ||||
| Texh (min) | Before | 0.0174 | 12.6 ± 3.5 | 12.6 ± 3.2 | 0.0318 |
| (11.0–14.2) | (11.1–14.1) | ||||
| After | 13.0 ± 3.4 | 13.3 ± 3.3 § | |||
| (11.4–14.6) | (11.8–14.8) | ||||
| Wmax (W) | Before | 0.1559 | 267 ± 72 | 267 ± 66 | 0.0951 |
| (234–299) | (237–297) | ||||
| After | 268 ± 66 | 276 ± 68 | |||
| (238–298) | (245–307) | ||||
| HRmax (bpm) | Before | 0.0000 | 182 ± 8 ‡‡ | 179 ± 8 | 0.2626 |
| (178–186) | (175–183) | ||||
| After | 180 ± 9 # | 182 ± 9 II §§ | |||
| (176–184) | (178–186) | ||||
| TGET (min) | Before | 0.0001 | 10.5 ± 2.5 | 10.6 ± 2.6 | 0.0097 |
| (9.4–11.7) | (9.4–11.8) | ||||
| After | 11.1 ± 2.8 ‡ | 11.1 ± 2.6 ¶ | |||
| (9.8–12.4) | (9.9–12.3) | ||||
| HRGET (bpm) | Before | 0.0785 | 171 ± 10 | 169 ± 10 | 0.2203 |
| (166–175) | (165–174) | ||||
| After | 172 ± 9 | 171 ± 9 | |||
| (168–176) | (167–175) | ||||
| WGET (W) | Before | 0.0000 | 226 ± 53 | 226 ± 52 | 0.0043 |
| (205–250) | (202–250) | ||||
| After | 236 ± 57 † | 240 ± 50 †† | |||
| (210–262) | (217–263) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durkalec-Michalski, K.; Zawieja, E.E.; Zawieja, B.E.; Jurkowska, D.; Buchowski, M.S.; Jeszka, J. Effects of Low Versus Moderate Glycemic Index Diets on Aerobic Capacity in Endurance Runners: Three-Week Randomized Controlled Crossover Trial. Nutrients 2018, 10, 370. https://doi.org/10.3390/nu10030370
Durkalec-Michalski K, Zawieja EE, Zawieja BE, Jurkowska D, Buchowski MS, Jeszka J. Effects of Low Versus Moderate Glycemic Index Diets on Aerobic Capacity in Endurance Runners: Three-Week Randomized Controlled Crossover Trial. Nutrients. 2018; 10(3):370. https://doi.org/10.3390/nu10030370
Chicago/Turabian StyleDurkalec-Michalski, Krzysztof, Emilia E. Zawieja, Bogna E. Zawieja, Dominika Jurkowska, Maciej S. Buchowski, and Jan Jeszka. 2018. "Effects of Low Versus Moderate Glycemic Index Diets on Aerobic Capacity in Endurance Runners: Three-Week Randomized Controlled Crossover Trial" Nutrients 10, no. 3: 370. https://doi.org/10.3390/nu10030370






