Beneficial Effect of Jojoba Seed Extracts on Hyperglycemia-Induced Oxidative Stress in RINm5f Beta Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Extraction of Plant Material
2.2. Identification and Quantification of Phenolic Compounds by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.3. Cell Culture and Treatment
2.4. Cell Viability and Functionality
2.5. ROS Production
2.6. Determination of Caspase-3 Activation
2.7. Protein Extraction and Western Blotting Analyses
2.8. Antioxidant Enzyme Activities
2.9. Statistical Analysis
3. Results
3.1. Characterization of the Phenolic Compounds from the Aqueous Jojoba Seed Extract
3.2. Cell Viability
3.3. Effect of Jojoba Extracts on Static Insulin Release
3.4. Jojoba Extracts Prevent Caspase-3 Activation and Oxidative Stress Induced by Fructose
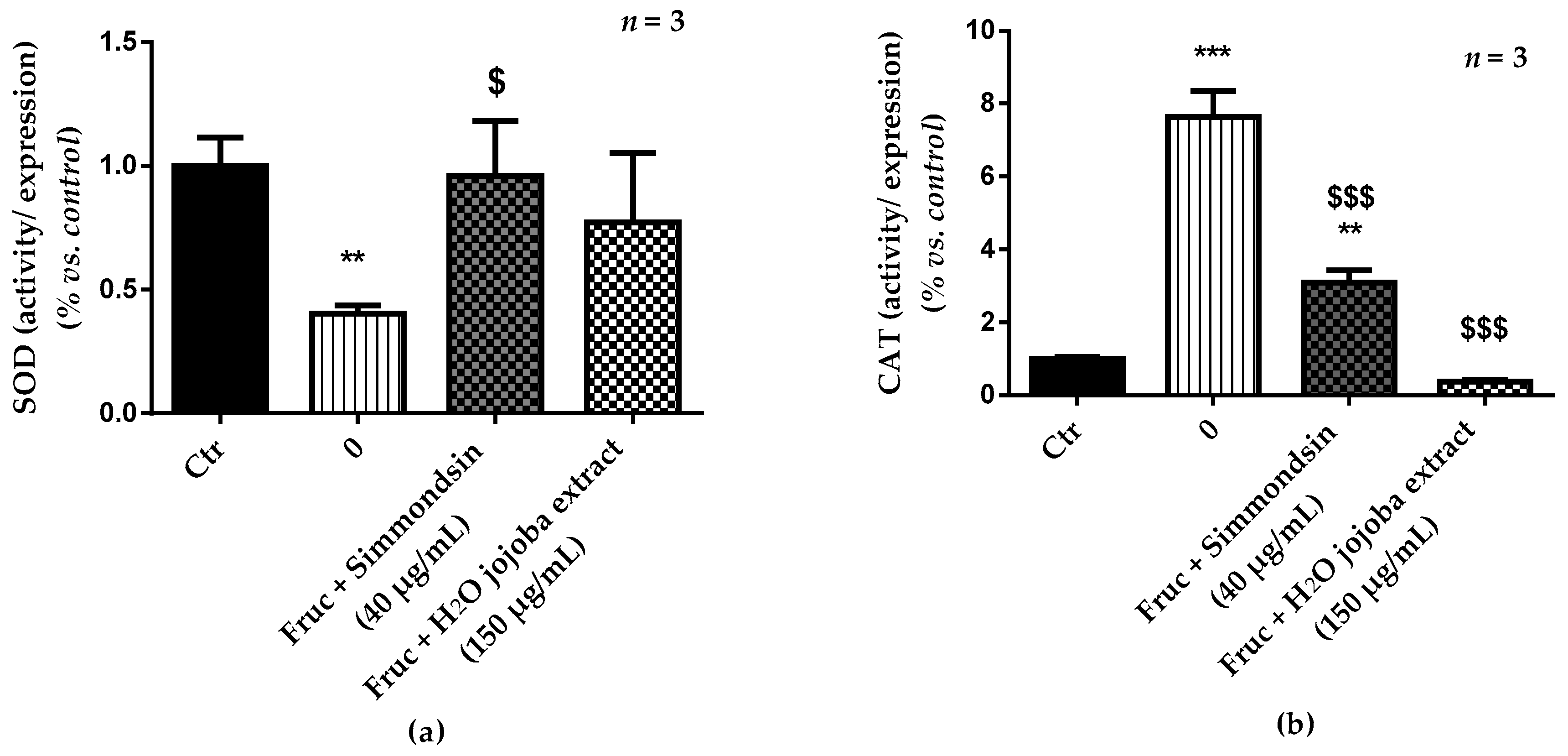
3.5. Enzymatic Antioxidant Defense Status and Oxidative Stress Damage
3.6. Effect of Jojoba Extracts on Pro-Oxidant Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IDF Diabetes Atlas-Home. Available online: http://www.diabetesatlas.org/ (accessed on 26 July 2017).
- Thomas, H.E.; Kay, T.W. Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes Metab. Res. Rev. 2000, 16, 251–261. [Google Scholar] [CrossRef]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. 2017, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta 2017, 1863, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar] [PubMed]
- Haskins, K.; Bradley, B.; Powers, K.; Fadok, V.; Flores, S.; Ling, X.; Pugazhenthi, S.; Reusch, J.; Kench, J. Oxidative stress in type 1 diabetes. Ann. N. Y. Acad. Sci. 2003, 1005, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U. Oxidative stress in vascular disease: Causes, defense mechanisms and potential therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Kreuzer, J. Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovasc. Res. 2005, 65, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hellerbrand, C.; Köhler, U.A.; Bugnon, P.; Kan, Y.-W.; Werner, S.; Beyer, T.A. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab. Investig. J. Tech. Methods Pathol. 2008, 88, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-J.; Zha, X.-J.; Kang, Z.-M.; Xu, M.-J.; Huang, Q.; Zou, D.-J. Therapeutic effects of hydrogen saturated saline on rat diabetic model and insulin resistant model via reduction of oxidative stress. Chin. Med. J. (Engl.) 2012, 125, 1633–1637. [Google Scholar] [PubMed]
- Grankvist, K.; Marklund, S.L.; Täljedal, I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981, 199, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.D.; Ghaskadbi, S.S. Islets and their antioxidant defense. Islets 2010, 2, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef]
- Sati, S.C.; Sati, N.; Rawat, U.; Sati, O.P. Medicinal Plants as a Source of Antioxidants. Res. J. Phytochem. 2010, 4, 213–224. [Google Scholar] [CrossRef]
- Parveen, A.; Rao, S. Effect of Nanosilver on Seed Germination and Seedling Growth in Pennisetum glaucum. J. Clust. Sci. 2015, 26, 693–701. [Google Scholar] [CrossRef]
- Akindele, A.J.; Adeyemi, O.O. Antiinflammatory activity of the aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia 2007, 78, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- A Natural History of the Sonoran Desert, 1st ed.; Phillips, S.J.; Comus, P.W. (Eds.) The University of California Press: Berkeley, CA, USA, 2000. [Google Scholar]
- Miwa, T.K. Jojoba oil wax esters and derived fatty acids and alcohols: Gas chromatographic analyses. J. Am. Oil Chem. Soc. 1971, 48, 259–264. [Google Scholar] [CrossRef]
- Abbott, T.P.; Holser, R.A.; Plattner, B.J.; Plattner, R.D.; Purcell, H.C. Pilot-scale isolation of simmondsin and related jojoba constituents. Ind. Crops Prod. 1999, 10, 65–72. [Google Scholar] [CrossRef]
- Elliger, C.A.; Waiss, A.C.; Lundin, R.E. Structure and stereochemistry of simmondsin. J. Org. Chem. 1974, 39, 2930–2931. [Google Scholar] [CrossRef]
- Van Boven, M.; Leyssen, T.; Busson, R.; Holser, R.; Cokelaere, M.; Flo, G.; Decuypere, E. Identification of 4,5-Didemethyl-4-O-α-d-glucopyranosylsimmondsin and Pinitol α-d-Galactosides in Jojoba Seed Meal (Simmondsia chinensis). J. Agric. Food Chem. 2001, 49, 4278–4283. [Google Scholar] [CrossRef] [PubMed]
- Lein, S.; Van Boven, M.; Holser, R.; Decuypere, E.; Flo, G.; Lievens, S.; Cokelaere, M. Simultaneous determination of carbohydrates and simmondsins in jojoba seed meal (Simmondsia chinensis) by gas chromatography. J. Chromatogr. A 2002, 977, 257–264. [Google Scholar] [CrossRef]
- Bellirou, A.; Bouali, A.; Bouammali, B.; Boukhatem, N.; Elmtili, B.N.; Hamal, A.; El-Mourabit, M. Extraction of simmondsin and oil in one step from jojoba seeds. Ind. Crops Prod. 2005, 21, 229–233. [Google Scholar] [CrossRef]
- Ogawa, K.; Watanabe, T.; Ikeda, Y.; Kondo, S. A new glycoside, 1d-2-O-α-d-galactopyranosyl-chiro-inositol from jojoba beans. Carbohydr. Res. 1997, 302, 219–221. [Google Scholar] [CrossRef]
- Yaron, A.; Samoiloff, V.; Benzioni, A. Absorption and distribution of orally administered jojoba wax in mice. Lipids 1982, 17, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, F.; Bernardi, M. Jojoba and Yucca; Ebury Press: London, UK, 1985; ISBN 978-0-7126-1008-7. [Google Scholar]
- Ranzato, E.; Martinotti, S.; Burlando, B. Wound healing properties of jojoba liquid wax: An in vitro study. J. Ethnopharmacol. 2011, 134, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M.M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Abou-Arab, A.A.; Salem, F.M.A. Antioxidant and antimicrobial effect of some natural plant extracts added to lamb patties during storage. Grasas Aceites 2011, 62, 139–148. [Google Scholar] [CrossRef]
- Wisniak, J. Potential uses of jojoba oil and meal—A review. Ind. Crops Prod. 1994, 3, 43–68. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; Shehata, A.H.; Sanad, O.A.; El-Latif, H.A.A. Biologically Active Flavonoids from Simmondsia chinensis (Link) Schneider Growing in Egypt. Available online: https://www.researchgate.net/publication/281594673_Biologically_active_flavonoids_from_Simmondsia_chinensis_Link_Schneider_growing_in_Egypt (accessed on 5 July 2017).
- Abbassy, M.A.; Abdelgaleil, S.A.M.; Belal, A.-S.H.; Rasoul, M.A.A.A. Insecticidal, antifeedant and antifungal activities of two glucosides isolated from the seeds of Simmondsia chinensis. Ind. Crops Prod. 2007, 26, 345–350. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Elliger, C.A.; Waiss, A.C.; Lundin, R.E. Simmondsin, an unusual 2-cyanomethylenecyclohexyl glucoside from Simmondsia californica. J. Chem. Soc. Perkin Trans. 1 1973, 2209–2212. [Google Scholar] [CrossRef]
- Verbiscar, A.J.; Banigan, T.F.; Weber, C.W.; Reid, B.L.; Trei, J.E.; Nelson, E.A.; Raffauf, R.F.; Kosersky, D. Detoxification of jojoba meal. J. Agric. Food Chem. 1980, 28, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Van Boven, M.; Busson, R.; Cokelaere, M.; Flo, G.; Decuypere, E. 4-Demethyl simmondsin from Simmondsia chinensis. Ind. Crops Prod. 2000, 12, 203–208. [Google Scholar] [CrossRef]
- Verbiscar, A.J. Jojoba Seed Meal as an Animal Feed; Anvers Bioscience Design, Inc.: Sierra Madre, CA, USA, 1982. [Google Scholar]
- Cokelaere, M.; Daenens, P.; Decuypere, E.; Flo, G.; Kühn, E.; Van Boven, M.; Vermaut, S. Reproductive performance of rats treated with defatted jojoba meal or simmondsin before or during gestation. Food Chem. Toxicol. 1998, 36, 13–19. [Google Scholar] [CrossRef]
- York, D.A.; Singer, L.; Oliver, J.; Abbott, T.P.; Bray, G.A. The detrimental effect of simmondsin on food intake and body weight of rats. Ind. Crops Prod. 2000, 12, 183–192. [Google Scholar] [CrossRef]
- Notoya, M.; Tsukamoto, Y.; Nishimura, H.; Woo, J.-T.; Nagai, K.; Lee, I.-S.; Hagiwara, H. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur. J. Pharmacol. 2004, 485, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pinent, S.M.J.; Romanowski, H.P.; Redaelli, L.R.; Cavalleri, A. Thysanoptera : Plantas visitadas e hospedeiras no Parque Estadual de Itapuã, Viamão, RS, Brasil. In Thysanoptera: Visited and Host Plants at Parque Estadual de Itapuã, Viamão, RS, Brazil; Instituto de Biociências: São Paulo, Brasil, 2005. (In Portuguese) [Google Scholar]
- Lizarraga, D.; Lozano, C.; Briedé, J.J.; van Delft, J.H.; Touriño, S.; Centelles, J.J.; Torres, J.L.; Cascante, M. The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts. FEBS J. 2007, 274, 4802–4811. [Google Scholar] [CrossRef] [PubMed]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2006, 27, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Harp, J.B. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, C807–C813. [Google Scholar] [CrossRef] [PubMed]
- Auberval, N.; Dal, S.; Bietiger, W.; Seyfritz, E.; Peluso, J.; Muller, C.; Zhao, M.; Marchioni, E.; Pinget, M.; Jeandidier, N.; et al. Oxidative Stress Type Influences the Properties of Antioxidants Containing Polyphenols in RINm5F Beta Cells. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Mol. Basel Switz. 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J. Biomed. Biotechnol. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Auger, C.; Alhosin, M.; Ebel, C.; Achour, M.; Etienne-Selloum, N.; Fuhrmann, G.; Bronner, C.; Schini-Kerth, V.B. Red wine polyphenols cause growth inhibition and apoptosis in acute lymphoblastic leukaemia cells by inducing a redox-sensitive up-regulation of p73 and down-regulation of UHRF1. Eur. J. Cancer Oxf. Engl. 2010, 46, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Fine, E.J. Fructose in perspective. Nutr. Metab. 2013, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Lanaspa, M.A.; Le, M.T.; Garcia, G.E.; Diggle, C.P.; Maclean, P.S.; Jackman, M.R.; Asipu, A.; Roncal-Jimenez, C.A.; Kosugi, T.; et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 4320–4325. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.D.; Stephenson, M.C.; Crossland, H.; Cordon, S.M.; Palcidi, E.; Cox, E.F.; Taylor, M.A.; Aithal, G.P.; Macdonald, I.A. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013, 145, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Susan, R.; Kathie, B.; Luann, J.; Matthew, P. Glycemic Effect of Nutritive Sweeteners: Honey, Sugar and High Fructose Corn Syrup. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=311847 (accessed on 6 July 2017).
- Ludwig, D.S. Dietary glycemic index and obesity. J. Nutr. 2000, 130, 280S–283S. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.; Abramson, J.L.; Vaccarino, V.; Gillespie, C.; Vos, M.B. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010, 303, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Longerich, L.; Gill, V. Prevention of fructose-induced hypertension by dietary vitamins. Clin. Biochem. 2004, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Massa, M.L.; Arbelaez, L.G.; Schinella, G.; Gagliardino, J.J.; Francini, F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015, 137, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Zhao, C.-H.; Yao, X.-L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Walker, M.D.; Kanner, J. Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J. Agric. Food Chem. 2002, 50, 7220–7225. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Huan, Y. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2008. [Google Scholar] [CrossRef]
- Ma, J.; Luo, X.-D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rai, G.K.; Upadhyay, A.K.; Kumar, R.; Singh, K.P. Antioxidant phytochemicals in tomato (Lycopersicon esculentum). Indian J. Agric. Sci. 2004, 74, 3–5. [Google Scholar]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Lin, J.-K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.; Virsolvy, A.; Quignard, J.F.; Ravier, M.A.; Bertrand, G.; Dalle, S.; Cros, G.; Magous, R.; Richard, S.; Oiry, C. Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells. Br. J. Pharmacol. 2013, 169, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim. Biophys. Acta 2017, 1863, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Ai, W.; Chakrabarti, M.; Banik, N.L.; Ray, S.K. KLF4 overexpression and apigenin treatment down regulated anti-apoptotic Bcl-2 proteins and matrix metalloproteinases to control growth of human malignant neuroblastoma SK-N-DZ and IMR-32 cells. Mol. Oncol. 2013, 7, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Kang, J.-S.; Park, J.H.Y.; Lee, Y.-J.; Choi, J.-S.; Kang, Y.-H. Polyphenolic Flavonoids Differ in Their Antiapoptotic Efficacy in Hydrogen Peroxide–Treated Human Vascular Endothelial Cells. J. Nutr. 2003, 133, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.Z. Recent natural products based drug development: A pharmaceutical industry perspective. J. Nat. Prod. 1998, 61, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The aging process: Major risk factor for disease and death. Proc. Natl. Acad. Sci. USA 1991, 88, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, M.; Francisco, G.; García-Arumí, E.; Hernández, C.; Martínez, R.; Andreu, A.L.; Simó, R. Mitochondrial DNA oxidation and manganese superoxide dismutase activity in peripheral blood mononuclear cells from type 2 diabetic patients. Diabetes Metab. 2008, 34, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Aouacheri, W.; Saka, S.; Djafer, R.; Lefranc, G. Protective effect of diclofenac towards the oxidative stress induced by paracetamol toxicity in rats. Ann. Biol. Clin. (Paris) 2009, 67, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, R.K.; Shinde, A.V.; Patil, S.M. Lipid profile, serum malondialdehyde, superoxide dismutase in chronic kidney diseases and Type 2 diabetes mellitus. Biomed. Res. 2012, 23, 2. [Google Scholar]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.E.; Ladiges, W.; Wolf, N.; Van Remmen, H.; et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Kang, M.-J.; Choi, H.-N.; Kim, J.-H.; Kim, J.-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Dinauer, M.C.; Pierce, E.A.; Bruns, G.A.; Curnutte, J.T.; Orkin, S.H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J. Clin. Investig. 1990, 86, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yuan, F.; Shen, X.; Wen, H.; Li, W.; Cheng, B.; Wu, J. Polymorphisms of C242T and A640G in CYBA Gene and the Risk of Coronary Artery Disease: A Meta-Analysis. PLoS ONE 2014, 9, e84251. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Khor, T.O.; Saw, C.L.L.; Loh, S.C.; Chen, A.I.; Lim, S.S.; Park, J.H.Y.; Cai, L.; Kong, A.-N.T. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; de Haan, J.B. Combating oxidative stress in diabetic complications with Nrf2 activators: How much is too much? Redox Rep. Commun. Free Radic. Res. 2014, 19, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]





| Peak No. | RT | % | Identified Compound |
|---|---|---|---|
| 1 | 3.2 | 36.60 | gallic acid |
| 2 | 4 | 4.46 | 3,5-dihydroxybenzoic acid |
| 3 | 20.6 | 1.12 | p-hydroxybenzoic acid |
| 4 | 23.8 | 1.12 | caffeic acid hexoside |
| 7 | 27.7 | 1.34 | syringic acid |
| 5 | 24.4 | 1.79 | catechin |
| 8 | 28.7 | 2.23 | kaempferol 3-glucoside |
| 10 | 29.8 | 1.12 | apigenin 7-rutinoside |
| 12 | 31.7 | 1.34 | quercetin-3,4-diglucoside |
| 15 | 34.3 | 1.12 | quercetin trisaccharide |
| 19 | 38.1 | 7.14 | rutin |
| 22 | 45.6 | 5.58 | epicatechin gallate |
| 23 | 50.5 | 1.12 | quercetin 3-glucoside |
| 24 | 59.1 | 4.46 | quercetin |
| 25 | 59.6 | 6.7 | isorhamnetin-3-glucoside |
| 9 | 29.2 | 1.56 | cyanidin-3-rutinoside |
| 6 | 25 | 1.56 | caffeic acid |
| 16 | 35.7 | 1.12 | p-coumaric acid |
| 20 | 41.5 | 3.79 | ferulic acid |
| 21 | 42.8 | 2.23 | sinapic acid |
| 11 | 31.3 | 0.89 | nd |
| 13 | 33.3 | 1.34 | nd |
| 14 | 33.9 | 0.89 | nd |
| 17 | 36.5 | 3.79 | nd |
| 18 | 37.5 | 1 .12 | nd |
| 26 | 61 | 2.90 | nd |
| 27 | 62 | 1.56 | nd |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhadj, S.; Hentati, O.; Hamdaoui, G.; Fakhreddine, K.; Maillard, E.; Dal, S.; Sigrist, S. Beneficial Effect of Jojoba Seed Extracts on Hyperglycemia-Induced Oxidative Stress in RINm5f Beta Cells. Nutrients 2018, 10, 384. https://doi.org/10.3390/nu10030384
Belhadj S, Hentati O, Hamdaoui G, Fakhreddine K, Maillard E, Dal S, Sigrist S. Beneficial Effect of Jojoba Seed Extracts on Hyperglycemia-Induced Oxidative Stress in RINm5f Beta Cells. Nutrients. 2018; 10(3):384. https://doi.org/10.3390/nu10030384
Chicago/Turabian StyleBelhadj, Sahla, Olfa Hentati, Ghaith Hamdaoui, Khaskhoussi Fakhreddine, Elisa Maillard, Stéphanie Dal, and Séverine Sigrist. 2018. "Beneficial Effect of Jojoba Seed Extracts on Hyperglycemia-Induced Oxidative Stress in RINm5f Beta Cells" Nutrients 10, no. 3: 384. https://doi.org/10.3390/nu10030384





