Dietary Sialyllactose Does Not Influence Measures of Recognition Memory or Diurnal Activity in the Young Pig
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Dietary Groups
2.3. Behavioral Testing
2.3.1. Novel Object Recognition
2.3.2. Activity Analysis
2.4. Statistical Analysis
3. Results
3.1. Diet Composition
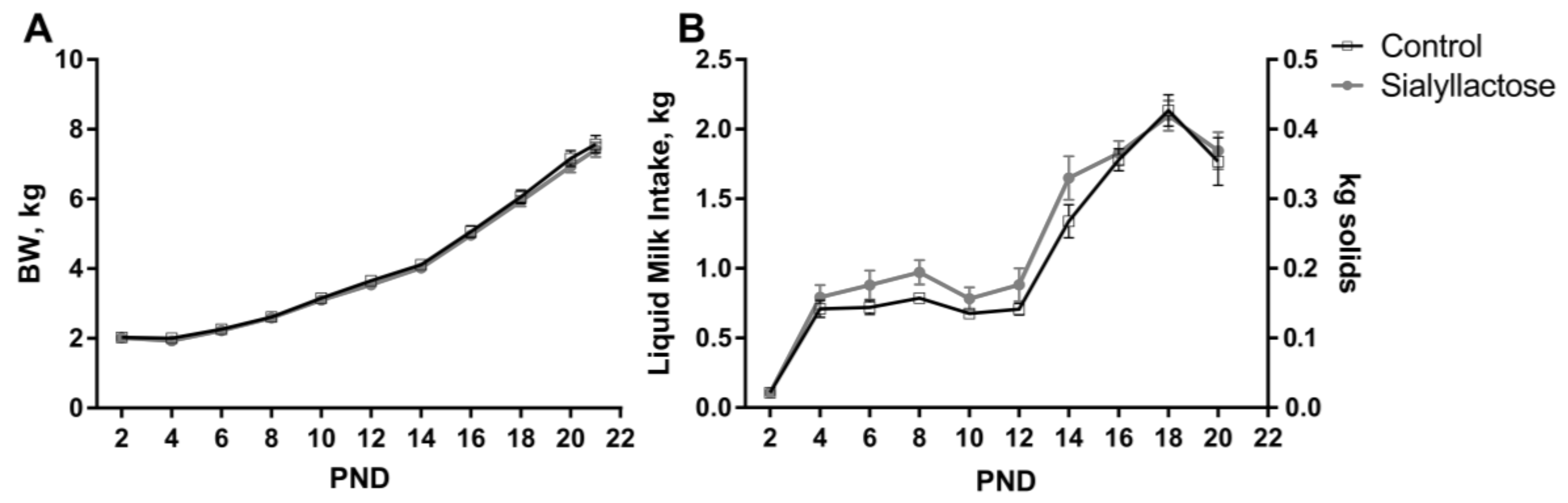
3.2. Growth Performance and Health Status
3.3. Novel Object Recognition
3.4. Activity Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; de Mola, C.L.; Victora, C.G. Breastfeeding and intelligence: A systematic review and meta-analysis. Acta Paediatr. Int. J. Paediatr. 2015, 104, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.-C.; Doyle, O.; Tremblay, R.E. Breastfeeding, Cognitive and Noncognitive Development in Early Childhood: A Population Study. Pediatrics 2017, 139, e20161848. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Boettcher, J.A.; Diersen-Schade, D.A. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: A review of randomized controlled trials. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Nutritional Importance of Choline for Brain Development. J. Am. Coll. Nutr. 2004, 23, 621S–626S. [Google Scholar] [CrossRef] [PubMed]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; McJarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Monaikul, S.; Patsavas, A.J.; Waworuntu, R.V.; Berg, B.M.; Dilger, R.N. Dietary polydextrose and galactooligosaccharide increase exploratory behavior, improve recognition memory, and alter neurochemistry in the young pig. Nutr. Neurosci. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krägeloh, C.U.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Radlowski, E.E.C.; Conrad, M.M.S.; Li, Y.; Dilger, R.N.; Johnson, R.W. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J. Nutr. 2014, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A.; Wang, B. Lactoferrin Promotes Early Neurodevelopment and Cognition in Postnatal Piglets by Upregulating the BDNF Signaling Pathway and Polysialylation. Mol. Neurobiol. 2015, 52, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3’Sialyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; Morairty, S.; Berg, B.M. Early life diet alters sleep architecture following an acute stress: The potential role of milk oligosaccharides. FASEB J. 2017, 31, 636. [Google Scholar]
- Sakai, F.; Ikeuchi, Y.; Urashima, T.; Fujihara, M.; Ohtsuki, K.; Yanahira, S. Effects of Feeding Sialyllactose and Galactosylated N-Acetylneuraminic Acid on Swimming Learning Ability and Brain Lipid Composition in Adult Rats. J. Appl. Glycosci. 2006, 53, 249–254. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [PubMed]
- Mudd, A.A.T.; Fleming, S.S.A.; Labhart, B.; Chichlowski, M.; Berg, B.B.M.; Donovan, S.S.M.; Dilger, R.R.N. Dietary Sialyllactose Influences Sialic Acid Concentrations in the Prefrontal Cortex and Magnetic Resonance Imaging Measures in Corpus Callosum of Young Pigs. Nutrients 2017, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Bruggencate, S.J.T.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Dilger, R.N. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav. Brain Res. 2017, 321, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sosa, S.; Martín, M.-J.; García-Pardo, L.A.; Hueso, P. Distribution of sialic acids in the milk of spanish mothers of full term infants during lactation. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine Milk Oligosaccharides and Sialic Acid Concentrations Vary throughout Lactation. Front. Nutr. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Spichtig, V.; Michaud, J.; Austin, S. Determination of sialic acids in milks and milk-based products. Anal. Biochem. 2010, 405, 28–40. [Google Scholar] [PubMed]
- Röhrig, C.H.; Choi, S.S.H.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, H.; Yu, B.; Troy, F.A. Molecular characterization of pig UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (Gne) gene: Effect of dietary sialic acid supplementation on gene expression in piglets. Curr. Top. Nutr. Res. 2007, 5, 165–175. [Google Scholar]
- Xu, X.; Zhu, T. Effect of ganglioside in repairing the neurological function of Chinese children with cerebral palsy. Chin. J. Clin. Rehabil. 2005, 9, 122–123. [Google Scholar]
- Neelima; Sharma, R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Fagioli, S.; Rossi-Arnaud, C.; Castellano, C. Dose-dependent effect of GM1 ganglioside during development on inhibitory avoidance behaviour in mice: Influence of the period of administration. Psychopharmacology (Berl.) 1992, 109, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.H.; Bellot, R.G.; Vital, M.A.; Frussa-Filho, R. Effects of long-term ganglioside GM1 administration on a new discriminative avoidance test in normal adult mice. Psychopharmacology (Berl.) 1997, 129, 322–328. [Google Scholar] [PubMed]
- Fong, T.G.; Neff, N.H.; Hadjiconstantinou, M. GM1 ganglioside improves spatial learning and memory of aged rats. Behav. Brain Res. 1997, 85, 203–211. [Google Scholar] [CrossRef]
- Silva, R.H.; Bergamo, M.; Frussa-Filho, R. Effects of neonatal ganglioside GM1 administration on memory in adult and old rats. Pharmacol. Toxicol. 2000, 87, 120–125. [Google Scholar] [CrossRef]
- Popov, N.; Toffano, G.; Riechert, U.; Matthies, H. Effects of intraventricularly applied gangliosides and N-acetylneuraminic acid on acquisition and retention performance of a brightness discrimination task in rats. Pharmacol. Biochem. Behav. 1989, 34, 209–212. [Google Scholar] [CrossRef]
- Silva, R.H.; Felicio, L.F.; Frussa-Filho, R. Ganglioside GM1 attenuates scopolamine-induced amnesia in rats and mice. Psychopharmacology (Berl.) 1999, 141, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Glasier, M.M.; Janis, L.S.; Goncalves, M.I.; Stein, D.G. GM1 produces attenuation of short-term memory deficits in Hebb-Williams maze performance after unilateral entorhinal cortex lesions. Physiol. Behav. 1999, 66, 441–446. [Google Scholar] [CrossRef]
- Morgan, B.L.; Winick, M. Effects of administration of N-acetylneuraminic acid (NANA) on brain NANA content and behavior. J. Nutr. 1980, 110, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.E.; Lomanowska, A.M.; McCutcheon, D.; Park, E.J.; Clandinin, M.T.; Ramanujam, K.S. Postnatal dietary supplementation with either gangliosides or choline: Effects on spatial short-term memory in artificially-reared rats. Nutr. Neurosci. 2007, 10, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, M.; Hodgkinson, S.C.; Fong, B.; Norris, C.; Guan, J.; Krageloh, C.U.; Breier, B.H.; Davison, M.; McJarrow, P.; Vickers, M.H. Maternal supplementation with a complex milk lipid mixture during pregnancy and lactation alters neonatal brain lipid composition but lacks effect on cognitive function in rats. Nutr. Res. 2010, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
| Nutrient per Liter | Control | Sialyllactose |
|---|---|---|
| Sialyllactose, mg | 58 | 374 |
| Energy and macronutrients | ||
| Total calories, kcal | 1049 | 1020 |
| Carbohydrate, g | 57 | 58 |
| Fat, g | 64 | 60 |
| Protein, g | 61 | 62 |
| Minerals | ||
| Calcium, mg | 2233 | 2178 |
| Chlorine, mg | 1141 | 1158 |
| Copper, μg | 1640 | 1505 |
| Iodine, μg | 274 | 271 |
| Iron, mg | 19 | 19 |
| Magnesium, mg | 227 | 241 |
| Manganese, μg | 2305 | 2159 |
| Phosphorus, mg | 1621 | 1673 |
| Potassium, mg | 2255 | 2349 |
| Selenium, μg | 65 | 68 |
| Sodium, mg | 1708 | 1708 |
| Zinc, mg | 17 | 17 |
| Vitamins and other nutrients | ||
| Vitamin A, IU | 4572 | 4112 |
| Vitamin D3, IU | 761 | 795 |
| Vitamin E, IU | 30 | 31 |
| Vitamin K, μg | 321 | 362 |
| Thiamin, μg | 1322 | 1588 |
| Riboflavin, μg | 2608 | 2780 |
| Niacin, μg | 13,366 | 11,132 |
| Vitamin B6, μg | 1210 | 1414 |
| Folic acid, μg | 211 | 237 |
| Vitamin B12, μg | 6 | 7 |
| Pantothenic acid, μg | 9216 | 8170 |
| Biotin, μg | 74 | 74 |
| Choline, mg | 352 | 394 |
| Polydextrose, g | 1.8 | 1.9 |
| Galactooligosaccharide, g | 2.1 | 1.7 |
| Arachidonic acid, mg | 318 | 288 |
| Docosahexaenoic acid, mg | 155 | 141 |
| Diet 2 | Pooled | |||
|---|---|---|---|---|
| Measure 3 | Control | Sialyllactose | SEM | p-Value 4 |
| ADG, g/day | 311 | 306 | 14 | 0.69 |
| ADMI, g milk/day | 1220 | 1347 | 62 | 0.11 |
| ADMI, g solids/day | 244 | 269 | 12 | 0.11 |
| G:F, g BW:kg milk | 255 | 234 | 11 | 0.16 |
| Diet | n | Mean | SEM | p-Value 2 |
|---|---|---|---|---|
| Control | 15 | 0.65 | 0.046 | <0.01 |
| Sialyllactose | 12 | 0.66 | 0.047 | <0.01 |
| Diet | ||||||
|---|---|---|---|---|---|---|
| Control | Sialyllactose | Pooled | ||||
| Measure | n | Mean | n | Mean | SEM | p-Value 2 |
| Recognition index | 15 | 0.66 | 12 | 0.65 | 0.05 | 0.94 |
| Novel object visit time, s | 15 | 56.63 | 12 | 42.05 | 7.77 | 0.18 |
| Number of novel object visits | 15 | 8.33 | 12 | 6.78 | 1.07 | 0.25 |
| Mean novel object visit time, s | 15 | 6.19 | 11 | 6.41 | 1.12 | 0.88 |
| Latency to first novel object visit, s | 15 | 25.46 | 12 | 25.32 | 9.31 | 0.99 |
| Habituation towards the novel object, s/min | 15 | −1.60 | 12 | −0.69 | 1.25 | 0.59 |
| Sample object visit time, s | 14 | 28.27 | 12 | 22.45 | 6.45 | 0.50 |
| Number of sample object visits | 15 | 4.77 | 12 | 4.62 | 0.56 | 0.84 |
| Mean sample object visit time, s | 15 | 6.18 | 12 | 5.51 | 1.34 | 0.71 |
| Latency to first sample object visit, s | 14 | 24.08 | 12 | 14.25 | 7.15 | 0.31 |
| Habituation towards the sample object, s/min | 14 | −2.68 | 12 | −1.78 | 0.73 | 0.35 |
| Total object visit time, s | 15 | 82.52 | 12 | 70.35 | 13.51 | 0.47 |
| Mean object visit time, s | 15 | 7.10 | 12 | 6.11 | 1.22 | 0.55 |
| Number of object visits | 15 | 13.13 | 12 | 11.42 | 1.34 | 0.31 |
| Latency to first object visit, s | 15 | 9.48 | 12 | 13.50 | 4.97 | 0.55 |
| Habituation towards both objects, s/min | 15 | −4.32 | 12 | −1.76 | 1.39 | 0.19 |
| Total distance moved, m | 15 | 2.43 | 11 | 2.11 | 0.19 | 0.11 |
| Time spent in the center of the arena, % | 15 | 58.76 | 12 | 56.73 | 6.95 | 0.80 |
| Diet 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Sialyllactose | |||||||||||
| Day | Night | Day | Night | Pooled | p-Value 3 | |||||||
| Measure | n | Mean | n | Mean | n | Mean | n | Mean | SEM | Diet | Cycle | Interaction |
| Total activity count | 65 | 1.6 × 105 | 70 | 6.9 × 104 | 67 | 1.7 × 105 | 72 | 6.9 × 104 | 7.0 × 103 | 0.64 | <0.01 | 0.56 |
| Time asleep, % | 65 | 67.47 | 70 | 84.90 | 71 | 67.43 | 72 | 85.63 | 0.97 | 0.61 | <0.01 | 0.58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, S.A.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary Sialyllactose Does Not Influence Measures of Recognition Memory or Diurnal Activity in the Young Pig. Nutrients 2018, 10, 395. https://doi.org/10.3390/nu10040395
Fleming SA, Chichlowski M, Berg BM, Donovan SM, Dilger RN. Dietary Sialyllactose Does Not Influence Measures of Recognition Memory or Diurnal Activity in the Young Pig. Nutrients. 2018; 10(4):395. https://doi.org/10.3390/nu10040395
Chicago/Turabian StyleFleming, Stephen A., Maciej Chichlowski, Brian M. Berg, Sharon M. Donovan, and Ryan N. Dilger. 2018. "Dietary Sialyllactose Does Not Influence Measures of Recognition Memory or Diurnal Activity in the Young Pig" Nutrients 10, no. 4: 395. https://doi.org/10.3390/nu10040395






