Ethanol Extract of Evodia rutaecarpa Attenuates Cell Growth through Caspase-Dependent Apoptosis in Benign Prostatic Hyperplasia-1 Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Plants, Chemicals, and Reagents
2.2. Preparation of a 70% Ethanol Extract of E. rutaecarpa (EEER)
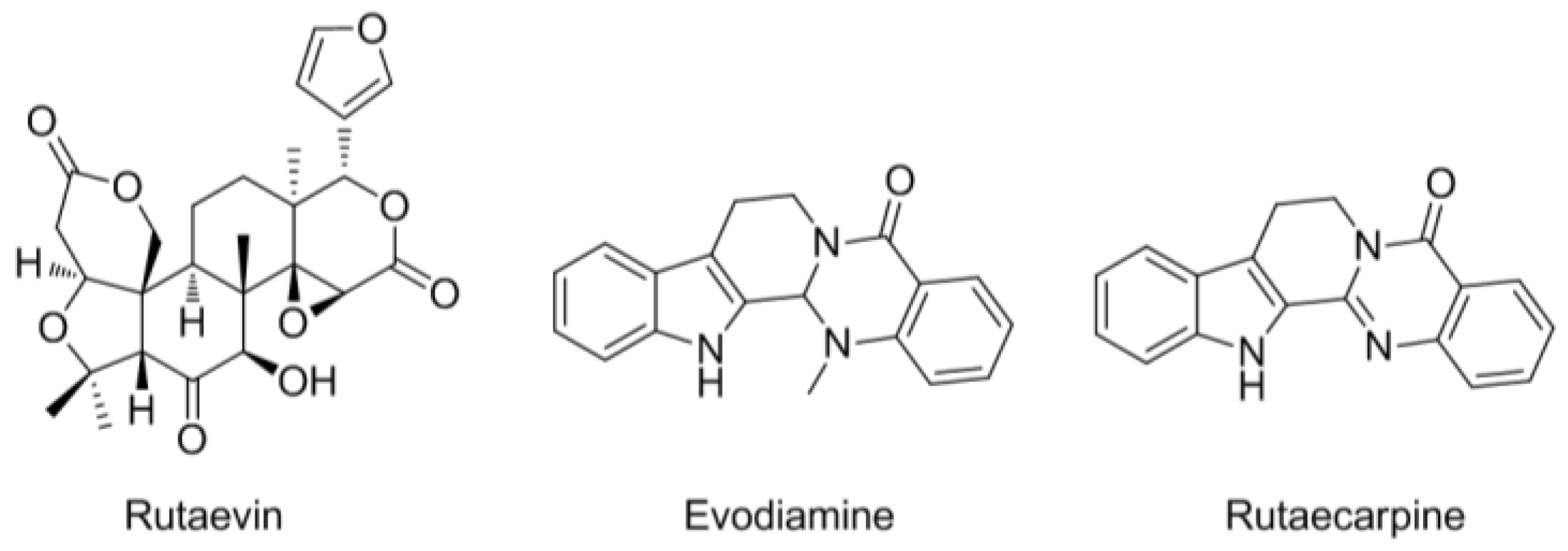
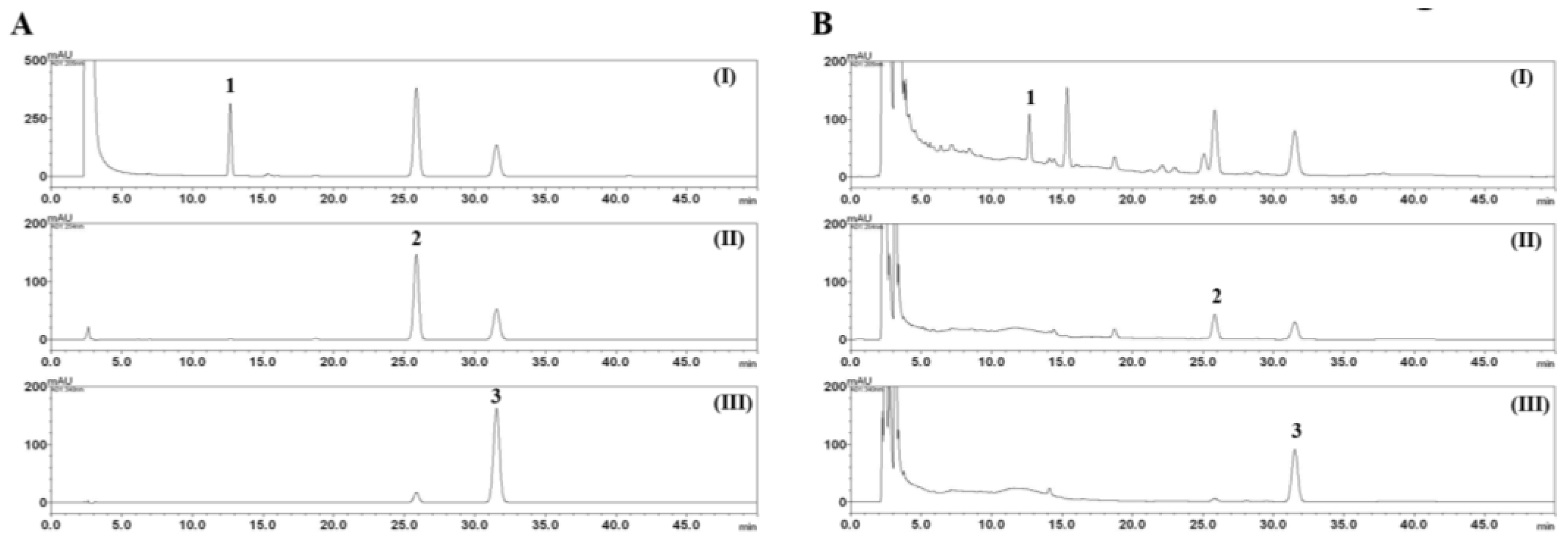
2.3. HPLC Analysis of the Three Marker Components of E. rutaecarpa
2.4. 5α-Reductase Activity
2.5. Cell Culture
2.6. Cell Growth Assay
2.7. Western Blot Analysis
2.8. DNA Fragmentation Assay
2.9. Nuclear Staining with DAPI
2.10. Determination of Caspase Activity
2.11. Statistical Analysis
3. Results
3.1. HPLC Analysis of the Three Marker Components of EEER
3.2. EEER Suppresses 5α-Reductase Activity
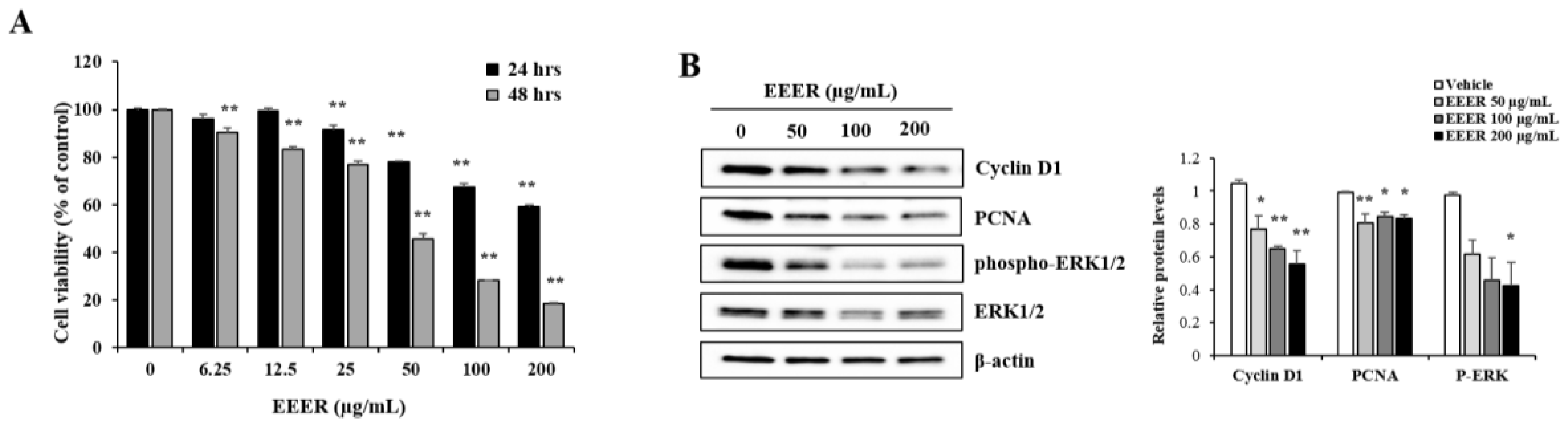
3.3. EEER Inhibits the Growth of BPH-1 Cells
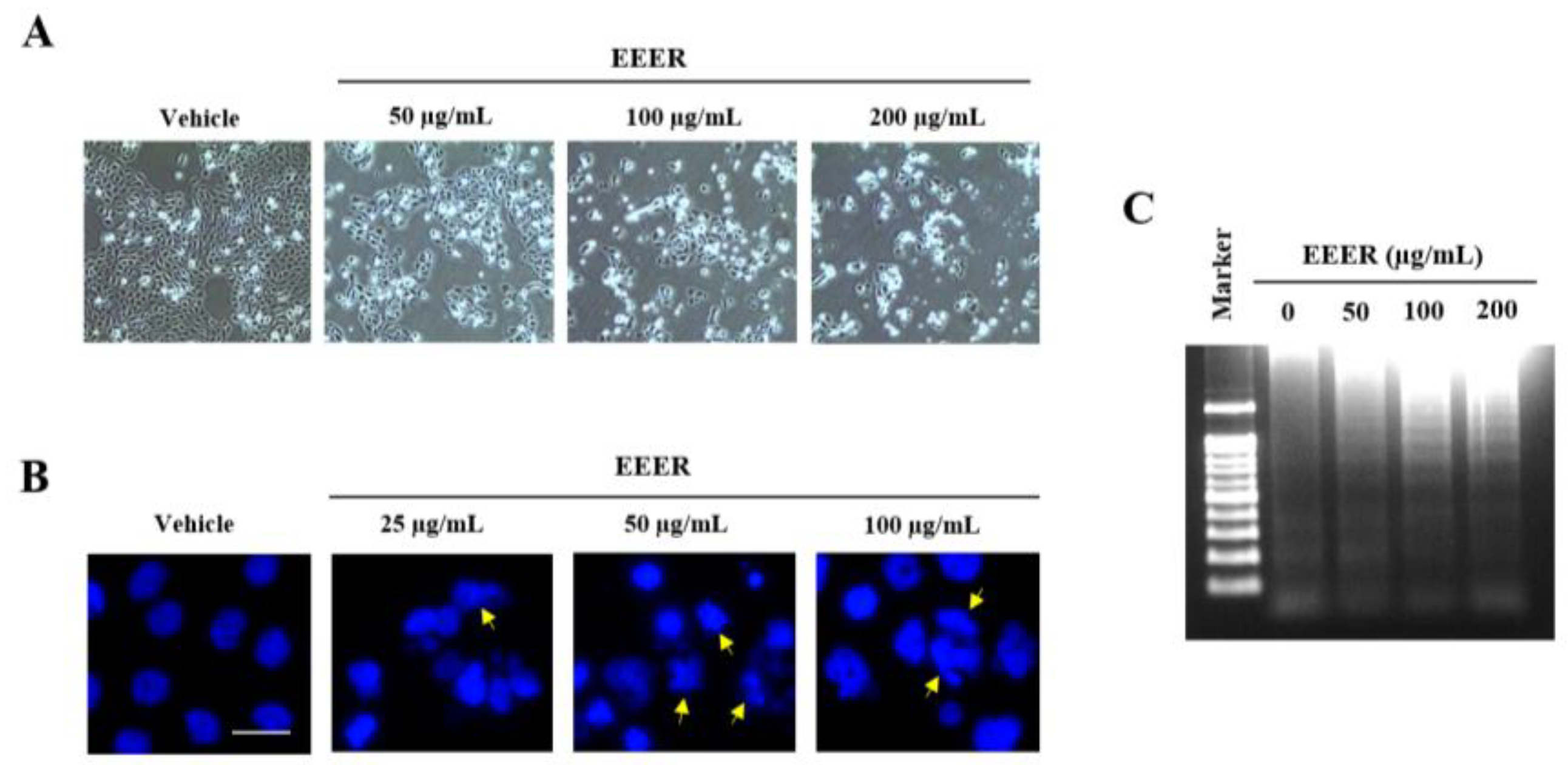
3.4. EEER Induces Apoptosis in BPH-1 Cells
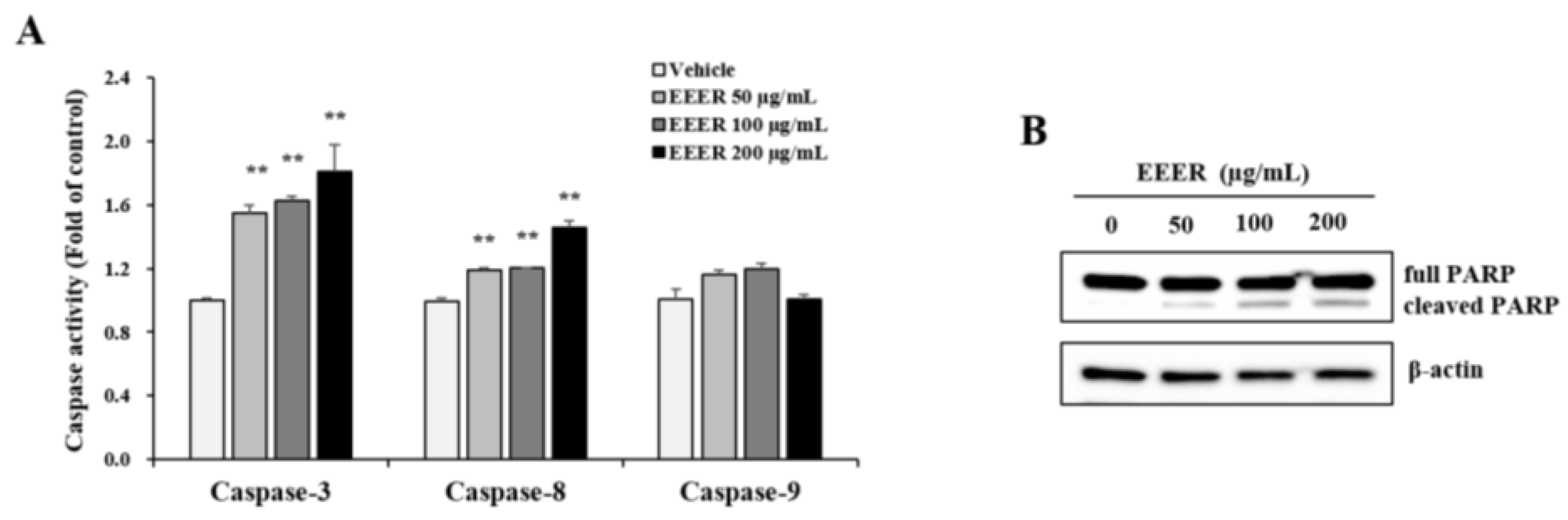
3.5. EEER-Induced Apoptosis Involves Caspase-8 and Caspase-3 Activation in BPH-1 Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, W.; Eisenbrand, G. Evodia rutaecarpa (juss.) benth. In Chinese Drugs of Plant Origin; Springer: Heidelberg, Germany, 1992; pp. 509–514. [Google Scholar]
- Yu, L.L.; Liao, J.F.; Chen, C.F. Anti-diarrheal effect of water extract of evodiae fructus in mice. J. Ethnopharmacol. 2000, 73, 39–45. [Google Scholar] [CrossRef]
- Yu, L.L.; Liao, J.F.; Chen, C.F. Effect of the crude extract of evodiae fructus on the intestinal transit in mice. Planta Med. 1994, 60, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Ko, H.C.; Chen, C.F.; Chou, C.J. Evodia rutaecarpa protects against circulation failure and organ dysfunction in endotoxaemic rats through modulating nitric oxide release. J. Pharm. Pharmacol. 2002, 54, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Wang, Y.H.; Liou, K.T.; Chen, C.M.; Chen, C.H.; Wang, W.Y.; Chang, S.; Hou, Y.C.; Chen, K.T.; Chen, C.F.; et al. Anti-inflammatory effects and mechanisms of the ethanol extract of evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur. J. Pharmacol. 2007, 555, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Shim, S.M.; Lee, S.R.; Mar, W.; Kim, G.H. Effect of evodiae fructus extracts on gene expressions related with alcohol metabolism and antioxidation in ethanol-loaded mice. Food Chem. Toxicol. 2005, 43, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, C.; Park, S.H.; Hong, S.H.; Kim, G.Y.; Hong, S.H.; Choi, Y.H. Induction of apoptosis by ethanol extract of evodia rutaecarpa in hela human cervical cancer cells via activation of amp-activated protein kinase. BioSci. Trends 2017, 10, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Oesterling, J.E. Benign prostatic hyperplasia: A review of its histogenesis and natural history. Prostate 1996, 29, 67–73. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E., Jr.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on aua guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Hsieh, P.; Barberia, J.; Pages, L.; Cosgrove, M. Altered blood androgens in elderly men with prostate hyperplasia. J. Clin. Endocrinol. Metab. 1975, 41, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Carson, C., 3rd; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Clark, R.V.; Hermann, D.J.; Cunningham, G.R.; Wilson, T.H.; Morrill, B.B.; Hobbs, S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J. Clin. Endocrinol. Metab. 2004, 89, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Imperato-McGinley, J.; McConnell, J.; Matsumoto, A.M.; Bracken, B.; Roy, J.; Sullivan, M.; Pappas, F.; Cook, T.; Daurio, C.; et al. Long-term (7 to 8-year) experience with finasteride in men with benign prostatic hyperplasia. Urology 2002, 60, 1040–1044. [Google Scholar] [CrossRef]
- Corona, G.; Tirabassi, G.; Santi, D.; Maseroli, E.; Gacci, M.; Dicuio, M.; Sforza, A.; Mannucci, E.; Maggi, M. Sexual dysfunction in subjects treated with inhibitors of 5alpha-reductase for benign prostatic hyperplasia: A comprehensive review and meta-analysis. Andrology 2017, 5, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Gilling, P.; Tammela, T.L.; Morrill, B.; Wilson, T.H.; Rittmaster, R.S. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: The enlarged prostate international comparator study (epics). BJU Int. 2011, 108, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chadha, R.; Dhingra, N. Phytotherapeutic agents for benign prostatic hyperplasia: An overview. Mini Rev. Med. Chem. 2017, 17, 1346–1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.U.; Ma, E. Synthesis and 5alpha-reductase inhibitory activity of c(2)(1) steroids having 1,4-diene or 4,6-diene 20-ones and 4-azasteroid 20-oximes. Molecules 2011, 17, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, M.Y.; Seo, C.S.; Jeon, W.Y.; Shin, H.K. Yongdamsagan-tang, a traditional herbal formula, inhibits cell growth through the suppression of proliferation and inflammation in benign prostatic hyperplasia epithelial-1 cells. J. Ethnopharmacol. 2017, 209, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef]
- Wang, K.; Fan, D.D.; Jin, S.; Xing, N.Z.; Niu, Y.N. Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian J. Androl. 2014, 16, 274–279. [Google Scholar] [PubMed]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot Res 2008, 20 (Suppl. 3), 11S–18S. [Google Scholar] [CrossRef] [PubMed]
- Stacey, D.W. Cyclin d1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr. Opin. Cell Biol. 2003, 15, 158–163. [Google Scholar] [CrossRef]
- Wang, S.C. Pcna: A silent housekeeper or a potential therapeutic target? Trends Pharmacol. Sci. 2014, 35, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lv, M.; Lin, H.; Cui, Y.; Wei, X.; Qin, Y.; Kohama, K.; Gao, Y. Rho-associated protein kinase isoforms stimulate proliferation of vascular smooth muscle cells through erk and induction of cyclin d1 and pcna. Biochem. Biophys. Res. Commun. 2013, 432, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Mittelman, A.; Serpico, R.; Sinigaglia, E.; Sinha, A.A.; Natale, C.; Santacroce, R.; Di Corcia, M.G.; Lucchese, A.; Dini, L.; et al. Cell death: Apoptosis versus necrosis (review). Int. J. Oncol. 2002, 21, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe 2010, 8, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Grootjans, S.; Goossens, V.; Dondelinger, Y.; Krysko, D.V.; Takahashi, N.; Vandenabeele, P. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 2013, 61, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(adp-ribose) polymerase (parp) cleavage in apoptosis. Caspase 3-resistant parp mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, Z.; Chen, Y.; Liu, Y.; Liu, Y.; Zhao, J.; Yi, J. Simultaneous determination of six bioactive compounds in evodiae fructus by high-performance liquid chromatography with diode array detection. J. Chromatogr. Sci. 2014, 52, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Son, J.K.; Chang, H.W.; Jahng, Y. Progress in studies on rutaecarpine. Ii.—Synthesis and structure-biological activity relationships. Molecules 2015, 20, 10800–10821. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Zhang, P.; Liu, X.M.; Du, Y.M.; Hou, X.D.; Cheng, S.; Zhang, Z.F. Cytological assessments and transcriptome profiling demonstrate that evodiamine inhibits growth and induces apoptosis in a renal carcinoma cell line. Sci. Rep. 2017, 7, 12572. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A novel anti-cancer alkaloid from evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.F.; Wang, B.X.; Li, T.J.; Tashiro, S.; Minami, M.; Xing, D.J.; Ikejima, T. Evodiamine, a constituent of evodiae fructus, induces anti-proliferating effects in tumor cells. Cancer Sci. 2003, 94, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Park, S.H.; Min, H.Y.; Park, H.J.; Lee, S.K. Anti-proliferative effects of evodiamine in human lung cancer cells. J. Cancer Prev. 2014, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.F.; Yu, C.H.; Pu, H.F.; Hsu, J.M.; Chen, M.J.; Wang, P.S. Anti-proliferative effects of evodiamine on human prostate cancer cell lines du145 and pc3. J. Cell. Biochem. 2007, 101, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.F.; Huang, W.J.; Lin, L.C.; Wang, P.S. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line lncap. Int. J. Cancer 2004, 110, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Jin, X.; Cao, Z.; Li, W. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase b signaling pathways. J. Tradit. Chin. Med. 2016, 36, 353–359. [Google Scholar] [PubMed]
- Zhang, C.; Fan, X.; Xu, X.; Yang, X.; Wang, X.; Liang, H.P. Evodiamine induces caspase-dependent apoptosis and s phase arrest in human colon lovo cells. Anticancer Drugs 2010, 21, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Yu, C.H.; Wang, S.W.; Pu, H.F.; Kan, S.F.; Lin, L.C.; Chi, C.W.; Ho, L.L.; Lee, C.H.; Wang, P.S. Anti-proliferative effects of evodiamine on human thyroid cancer cell line aro. J. Cell. Biochem. 2010, 110, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Concentrations | ||
|---|---|---|---|
| Inhibition (%) at a Concentration of Extract | |||
| Finasteride | 0.372 ng/mL | 3.72 ng/mL | 37.2 ng/mL |
| 20.1 ± 14.6 | 68.6 ± 4.8 | 89.3 ± 15.4 | |
| EEER | 25 μg/mL | 100 μg/mL | 250 μg/mL |
| 80.2 ± 11.4 | 94.3 ± 10.7 | 104.1 ± 4.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Lee, M.-Y.; Seo, C.-S.; Jang, J.-H.; Kim, Y.-u.; Shin, H.-K. Ethanol Extract of Evodia rutaecarpa Attenuates Cell Growth through Caspase-Dependent Apoptosis in Benign Prostatic Hyperplasia-1 Cells. Nutrients 2018, 10, 523. https://doi.org/10.3390/nu10040523
Park E, Lee M-Y, Seo C-S, Jang J-H, Kim Y-u, Shin H-K. Ethanol Extract of Evodia rutaecarpa Attenuates Cell Growth through Caspase-Dependent Apoptosis in Benign Prostatic Hyperplasia-1 Cells. Nutrients. 2018; 10(4):523. https://doi.org/10.3390/nu10040523
Chicago/Turabian StylePark, Eunsook, Mee-Young Lee, Chang-Seob Seo, Ji-Hye Jang, Yong-ung Kim, and Hyeun-Kyoo Shin. 2018. "Ethanol Extract of Evodia rutaecarpa Attenuates Cell Growth through Caspase-Dependent Apoptosis in Benign Prostatic Hyperplasia-1 Cells" Nutrients 10, no. 4: 523. https://doi.org/10.3390/nu10040523
APA StylePark, E., Lee, M. -Y., Seo, C. -S., Jang, J. -H., Kim, Y. -u., & Shin, H. -K. (2018). Ethanol Extract of Evodia rutaecarpa Attenuates Cell Growth through Caspase-Dependent Apoptosis in Benign Prostatic Hyperplasia-1 Cells. Nutrients, 10(4), 523. https://doi.org/10.3390/nu10040523





