TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blot
2.3. Mg2+ Influx Measurements
2.4. Cell Cycle Analysis
2.5. Scratch Assay
2.6. Statistical Analysis
3. Results
3.1. Cell Characterization
3.2. Contribution of TRPM7 to Colon Cell Functions
3.3. Contribution of TRPM6 to Colon Cell Functions
3.4. Contribution of TRPM6/7 Channels
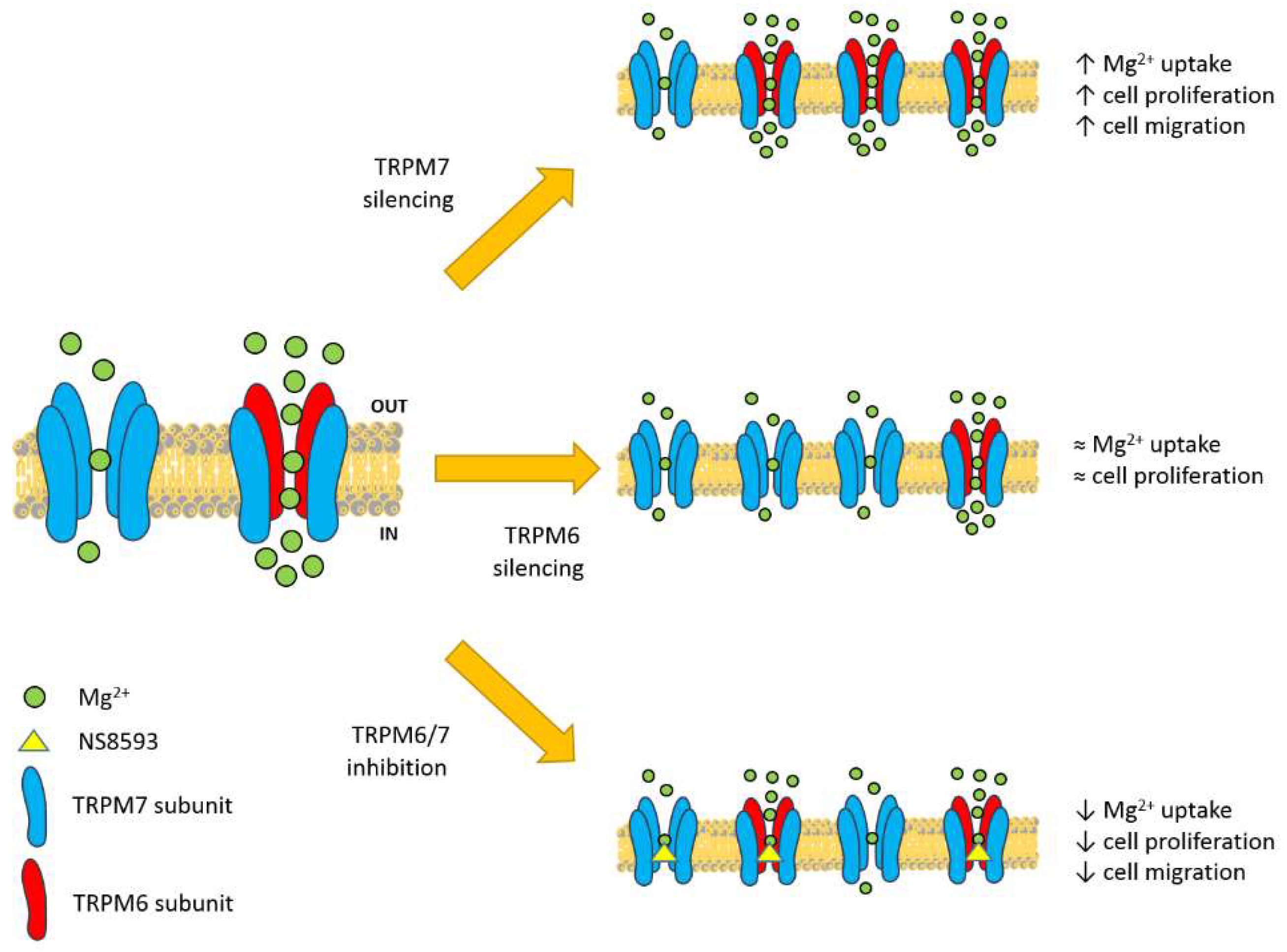
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.J.; Ritchie, G.; Kerstan, D.; Kang, H.S.; Cole, D.E.; Quamme, G.A. Magnesium transport in the renal distal convoluted tubule. Physiol. Rev. 2001, 81, 51–84. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008, 24, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010, 298, C407–C429. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Mittermeier, L.; Gudermann, T. Role of kinase-coupled TRP channels in mineral homeostasis. Pharmacol. Ther. 2018, 184, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Perraud, A.L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [PubMed]
- Groenestege, W.M.; Hoenderop, J.G.; van den Heuvel, L.; Knoers, N.; Bindels, R.J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 2006, 17, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Lameris, A.L.; Nevalainen, P.I.; Reijnen, D.; Simons, E.; Eygensteyn, J.; Monnens, L.; Bindels, R.J.; Hoenderop, J.G. Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G206–G216. [Google Scholar] [CrossRef] [PubMed]
- Rondón, L.J.; Groenestege, W.M.; Rayssiguier, Y.; Mazur, A. Relationship between low magnesium status and TRPM6 expression in the kidney and large intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R2001–R2007. [Google Scholar] [CrossRef] [PubMed]
- Van Angelen, A.A.; San-Cristobal, P.; Pulskens, W.P.; Hoenderop, J.G.; Bindels, R.J. The impact of dietary magnesium restriction on magnesiotropic and calciotropic genes. Nephrol. Dial. Transplant. 2013, 28, 2983–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Nejsum, L.N.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Walder, R.Y.; Landau, D.; Meyer, P.; Shalev, H.; Tsolia, M.; Borochowitz, Z.; Boettger, M.B.; Beck, G.E.; Englehardt, R.K.; Carmi, R.; Sheffield, V.C. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002, 31, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Ferioli, S.; Wisnowsky, A.; Simmons, D.G.; Leitzinger, C.; Einer, C.; Jonas, W.; Shymkiv, Y.; Bartsch, H.; Braun, A.; et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife 2016, 5, e20914. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Waldegger, S.; Mederos y Schnitzler, M.; Vitzthum, H.; Sassen, M.C.; Seyberth, H.W.; Konrad, M.; Gudermann, T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 2004, 101, 2894–2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, C.; Dorovkov, M.V.; Zhao, X.; Davenport, B.J.; Ryazanov, A.G.; Perraud, A.L. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J. Biol. Chem. 2005, 280, 37763–37771. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, J.; Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Petito, V.; Di Agostini, A.; Arduini, D.; Hamersma, W.; Pietropaolo, G.; Luongo, F.; Arena, V.; Stigliano, E.; Lopetuso, L.R.; et al. Dietary magnesium alleviates experimental murine colitis through upregulation of the transient receptor potential melastatin 6 channel. Inflamm. Bowel Dis. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Arduini, D.; Luongo, F.; Wolf, F.I. EGF stimulates Mg2+ influx in mammary epithelial cells. Biochem. Biophys. Res. Commun. 2014, 454, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Schweigel-Röntgen, M.; Cittadini, A.; Wolf, F.I. Intracellular magnesium detection by fluorescent indicators. Methods Enzymol. 2012, 505, 421–444. [Google Scholar] [PubMed]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Boninsegna, A.; Mazur, A.; Maier, J.A. Magnesium deficiency affects mammary epithelial cell proliferation: Involvement of oxidative stress. Nutr. Cancer 2009, 61, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Mederos y Schnitzler, M.; Meißner, M.; Schäfer, S.; Abstiens, K.; Hofmann, T.; Gudermann, T. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 2012, 166, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, S.; Zierler, S.; Zaißerer, J.; Schredelseker, J.; Gudermann, T.; Chubanov, V. TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP. Sci. Rep. 2017, 7, 8806. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Arduini, D.; Cittadini, A.; Wolf, F.I. From magnesium to magnesium transporters in cancer: TRPM7, a novel signature in tumour development. Magnes. Res. 2013, 26, 149–155. [Google Scholar] [PubMed]
- Gautier, M.; Perrière, M.; Monet, M.; Vanlaeys, A.; Korichneva, I.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H. Recent advances in oncogenic roles of the TRPM7 chanzyme. Curr. Med. Chem. 2016, 23, 4092–4107. [Google Scholar] [CrossRef] [PubMed]
- Deason-Towne, F.; Perraud, A.L.; Schmitz, C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011, 585, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Moscheni, C.; Trapani, V.; Wolf, F.I.; Farruggia, G.; Sargenti, A.; Iotti, S.; Maier, J.A.; Castiglioni, S. The different expression of TRPM7 and MagT1 impacts on the proliferation of colon carcinoma cells sensitive or resistant to doxorubicin. Sci. Rep. 2017, 7, 40538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yu, H.; Huang, J.; Faouzi, M.; Schmitz, C.; Penner, R.; Fleig, A. The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels. J. Biol. Chem. 2014, 289, 5217–5227. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Schlingmann, K.P.; Wäring, J.; Heinzinger, J.; Kaske, S.; Waldegger, S.; Mederos y Schnitzler, M.; Gudermann, T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 2007, 282, 7656–7667. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, L.V.; Hu, Z.M.; Suzuki, S.; Chubanov, V.; Fleig, A.; Ryazanov, A.G. Elucidating the role of the TRPM7 alpha-kinase: TRPM7 kinase inactivation leads to magnesium deprivation resistance phenotype in mice. Sci. Rep. 2014, 4, 7599. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.I.; Trapani, V. TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon. Nutrients 2018, 10, 784. https://doi.org/10.3390/nu10060784
Luongo F, Pietropaolo G, Gautier M, Dhennin-Duthille I, Ouadid-Ahidouch H, Wolf FI, Trapani V. TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon. Nutrients. 2018; 10(6):784. https://doi.org/10.3390/nu10060784
Chicago/Turabian StyleLuongo, Francesca, Giuseppe Pietropaolo, Mathieu Gautier, Isabelle Dhennin-Duthille, Halima Ouadid-Ahidouch, Federica I. Wolf, and Valentina Trapani. 2018. "TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon" Nutrients 10, no. 6: 784. https://doi.org/10.3390/nu10060784




