Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements
Abstract
:1. Introduction
2. Methods
Data Extraction
3. Results
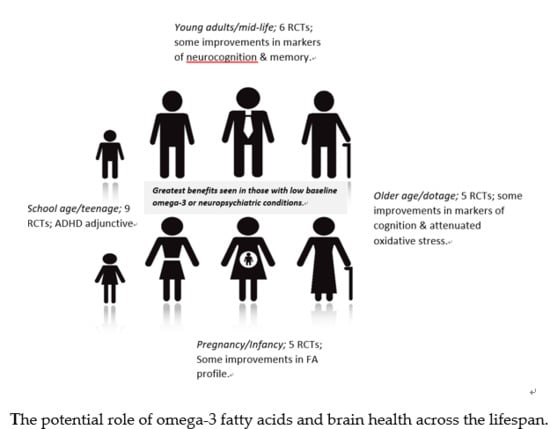
3.1. Pregnancy and Infancy
3.2. School Age and Teenage
3.3. Young Adults
3.4. Middle Age
3.5. Older Age/Dotage
4. Discussion
Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclosure
References
- What is brain health? Available online: https://brainhealth.nia.nih.gov/ (accessed on 8 October 2018).
- What is a Healthy Brain? New Research Explores Perceptions of Cognitive Health Among Diverse Older Adults. Available online: https://www.cdc.gov/aging/pdf/Perceptions_of_Cog_Hlth_factsheet.pdf (accessed on 8 October 2018).
- Gorelick, P.B.; Furie, K.L.; Iadecola, C.; Smith, E.E.; Waddy, S.P.; Lloyd-Jones, D.M.; Bae, H.-J.; Bauman, M.A.; Dichgans, M.; Duncan, P.W.; et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke 2017, 48, e284–e303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dementia and cognitive decline: A review of the evidence. Available online: https://www.ageuk.org.uk/documents/EN-GB/For-professionals/Research/Cognitive_decline_and_dementia_evidence_review_Age_UK.pdf?dtrk=true (accessed on 8 October 2018 ).
- Poddar, J.; Pradhan, M.; Ganguly, G.; Chakrabarti, S. Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements. J. Chem. Neuroanat. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dementia UK Second Edition. Available online: http://eprints.lse.ac.uk/59437/1/Dementia_UK_Second_edition_-_Overview.pdf (accessed on 8 October 2018).
- Dementia Statistics. Available online: https://www.alz.co.uk/research/statistics (accessed on 8 October 2018).
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Chiu, C.C.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J. Clin. Psychiatry 2012, 73, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; Duan, L.L.; Erskine, H.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Heart Attack and Stroke Symptoms. What is brain health? Available online: https://www.heart.org/en/health-topics/brain-health (accessed on 8 October 2018).
- Karr, J.E.; Graham, R.B.; Hofer, S.M.; Muniz-Terrera, G. When does cognitive decline begin? A systematic review of change point studies on accelerated decline in cognitive and neurological outcomes preceding mild cognitive impairment, dementia, and death. Psychol. Aging 2018, 33, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; da Costa Ferreira, G.; Trevenzoli, I.H.; de Jesus Oliveira, K.; de Melo Reis, R.A. Fatty Acids, Antioxidants and Physical Activity in Brain Aging. Nutrients 2017, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimers Dement. 2017, 3, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance-A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Can. Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Omega-3 polyunsaturated fatty acids and non-communicable diseases: Meta-analysis based systematic review. Asia Pac. J. Clin. Nutr. 2015, 24, 10–15. [Google Scholar] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Guest, J. Role of Omega-3 PUFAs in Neurobiological Health. Adv. Neurobiol. 2016, 12, 247–274. [Google Scholar] [PubMed]
- Denis, I.; Potier, B.; Vancassel, S.; Heberden, C.; Lavialle, M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Res. Rev. 2013, 12, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Parellada, M.; Llorente, C.; Calvo, R.; Gutierrez, S.; Lázaro, L.; Graell, M.; Dorado, M.L.; Boada, L.; Romo, J.; Dulin, E.; et al. Randomized trial of omega-3 for autism spectrum disorders: Effect on cell membrane composition and behavior. Eur. Neuropsychopharmacol. 2017, 27, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Brew, B.K.; Toelle, B.G.; Webb, K.L.; Almqvist, C.; Marks, G.B. Omega-3 supplementation during the first 5 years of life and later academic performance: A randomised controlled trial. Eur. J. Clin. Nutr. 2015, 69, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Diepen, R.M.V.; Weusten, J.M.H.; Demmelmair, H.; Koletzko, B.; de Sain-van der Velden, M.G.M.; Eilander, A.; Hoeksma, M.; et al. Reduced Symptoms of Inattention after Dietary Omega-3 Fatty Acid Supplementation in Boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Mulder, K.A.; King, D.J.; Innis, S.M. Omega-3 fatty acid deficiency in infants before birth identified using a randomized trial of maternal DHA supplementation in pregnancy. PLoS ONE 2014, 9, e83764. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Makrides, M.; Colombo, J.; Smithers, L.G. Randomized controlled trial of maternal omega-3 long-chain PUFA supplementation during pregnancy and early childhood development of attention, working memory, and inhibitory control. Am. J. Clin. Nutr. 2014, 99, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado, J.A.; Iznaola, C.; Peña, M.; Ruíz, J.; Peña-Quintana, L.; Kajarabille, N.; Rodriguez-Santana, Y.; Sanjurjo, P.; Aldámiz-Echevarría, L.; Ochoa, J. Effects of Maternal Omega-3 Supplementation on Fatty Acids and on Visual and Cognitive Development. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Milte, C.M.; Parletta, N.; Buckley, J.D. Increased Erythrocyte Eicosapentaenoic Acid and Docosahexaenoic Acid Are Associated With Improved Attention and Behavior in Children With ADHD in a Randomized Controlled Three-Way Crossover Trial. J. Atten. Disord. 2015, 19, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins, Leukot. Essent. Fatty Acids 2015, 95, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Young, A.S.; Belury, M.A.; Cole, R.M.; Gracious, B.; Seidenfeld, A.M.; Wolfson, H.; Fristad, M.A. Omega-3 Fatty Acid Plasma Levels Before and After Supplementation: Correlations with Mood and Clinical Outcomes in the Omega-3 and Therapy Studies. J. Child Adolesc. Psychopharmacol. 2017, 27, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, P.; Spreckelsen, T.F.; Burton, A.; Burton, J.R.; Richardson, A.J. Docosahexaenoic acid for reading, working memory and behavior in UK children aged 7–9: A randomized controlled trial for replication (the DOLAB II study). PLoS ONE 2018, 13, e0192909. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Fransson, G.; Östlund, S.; Areskoug, B.; Gillberg, C. Omega 3/6 fatty acids for reading in children: A randomized, double-blind, placebo-controlled trial in 9-year-old mainstream schoolchildren in Sweden. J. Child Psychol. Psychiatry 2017, 58, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Reyes, V.; Pérez-García, M.; Loya-Méndez, Y.; Puente, A.E. Clinical significance of neuropsychological improvement after supplementation with omega-3 in 8-12 years old malnourished Mexican children: A randomized, double-blind, placebo and treatment clinical trial. Res. Dev. Disabil. 2014, 35, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Niyonsenga, T.; Duff, J. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Levels and Correlations with Symptoms in Children with Attention Deficit Hyperactivity Disorder, Autistic Spectrum Disorder and Typically Developing Controls. PLoS ONE 2016, 11, e0156432. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.E.; Mahoney, C.R.; Urry, H.L.; Brunyé, T.T.; Taylor, H.A.; Kanarek, R.B. Omega-3 fatty acids and stress-induced changes to mood and cognition in healthy individuals. Pharmacol. Biochem. Behav. 2015, 132, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Cooper, P.; Gent, D.N.; Petkov, J.; O’Dea, K. Effects of fish oil supplementation on learning and behaviour of children from Australian Indigenous remote community schools: A randomised controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kulzow, N.; Veronica, W.A.; Lucia, K.; Ulrike, G.; Jan Philipp, S.; Andreas, H.; Agnes, F. Impact of Omega-3 Fatty Acid Supplementation on Memory Functions in Healthy Older Adults. J. Alzheimers Dis. 2016, 51, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does consumption of LC omega-3 PUFA enhance cognitive performance in healthy school-aged children and throughout adulthood? Evidence from clinical trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. 2014, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Jaremka, L.M.; Derry, H.M.; Bornstein, R.; Prakash, R.S.; Peng, J.; Belury, M.A.; Andridge, R.R.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Omega-3 supplementation and loneliness-related memory problems: Secondary analyses of a randomized controlled trial. Psychosom. Med. 2014, 76, 650–658. [Google Scholar] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 2014, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Widenhorn-Muller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 49–60. [Google Scholar]
- Hooper, C.; de Souto Barreto, P.; Coley, N.; Cantet, C.; Cesari, M.; Andrieu, S.; Vellas, B.; MAPT/DSA Study Group. Cognitive Changes with Omega-3 Polyunsaturated Fatty Acids in Non-Demented Older Adults with Low Omega-3 Index. J. Nutr. Health Aging 2017, 21, 988–993. [Google Scholar]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 Polyunsaturated Fatty Acids Supplementation Improved the Cognitive Function in the Chinese Elderly with Mild Cognitive Impairment: A Double-Blind. Randomized Controlled Trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Boespflug, E.L.; McNamara, R.K.; Eliassen, J.C.; Schidler, M.D.; Krikorian, R. Fish. Oil Supplementation Increases Event-Related Posterior Cingulate Activation in Older Adults with Subjective Memory Impairment. J. Nutr. Health Aging 2016, 20, 161–169. [Google Scholar] [PubMed]
- Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin. Interv. Aging 2013, 8, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.L.; Lagopoulos, J.; Cockayne, N.; Lewis, S.J.G.; Hickie, L.B.; Hermens, D.F.; Naismith, S.L. The effect of 12-wk omega-3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients "at risk" for major depression. Nutrition 2015, 31, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 Polyunsaturated Fatty Acid Intake in European Countries in Light of the Current Recommendations-Focus on Specific Population Groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An. Analysis of NHANES 2001-2014. Nutrients 2018, 10, 416. [Google Scholar]
- Cairncross, M.; Miller, C.J. The Effectiveness of Mindfulness-Based Therapies for ADHD: A Meta-Analytic Review. J. Atten. Disord. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pinar, C.; Martínez-Sanchís, S.; Carbonell-Vayá, E.; Fenollar-Cortés, J.; Sánchez-Meca, J. Long-Term Efficacy of Psychosocial Treatments for Adults With Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Front. Psychol. 2018, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Berk, L.; Hotterbeekx, R.; van Os, J.; van Boxtel, M. Mindfulness-based stress reduction in middle-aged and older adults with memory complaints: A mixed-methods study. Aging Ment Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; Kelly, P.; Stanton, C.; Cryan, J.F.; Dinan, T.G. N-3 Polyunsaturated Fatty Acids through the Lifespan: Implication for Psychopathology. Int. J. Neuropsychopharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.E.; Alexander, J.E.; Winningham, R.G. Omega-3 polyunsaturated fatty acids and cognition throughout the lifespan: A review. Nutr. Neurosci. 2011, 14, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The Omega-6: Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jerneren, F.; Elshorbagy, A.K.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith, A.D. Brain atrophy in cognitively impaired elderly: The importance of long-chain omega-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.E.; Kubow, S.; Egeland, G.M. Is iron status associated with highly unsaturated fatty acid status among Canadian Arctic Inuit? Food Funct. 2011, 2, 381–385. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Preventing Dementia and Cognitive Impairment; Leshner, A.I.; Landis, S. Preventing Cognitive Decline and Dementia: A Way Forward; Downey, A., Stroud, C., Eds.; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Wasay, M.; Grisold, W.; Carroll, W.; Shakir, R. World Brain Day 2016: Celebrating brain health in an ageing population. Lancet Neurol. 2016, 15, 1008. [Google Scholar] [CrossRef]
- Watanabe, N.; Furukawa, T.A.; Horikoshi, M.; Katsuki, F.; Narisawa, T.; Kumachi, M.; Oe, Y.; Shimei, I.; Noguchi, H.; Hamazaki, K.; et al. A mindfulness-based stress management program and treatment with omega-3 fatty acids to maintain a healthy mental state in hospital nurses (Happy Nurse Project): Study protocol for a randomized controlled trial. Trials 2015, 16, 36. [Google Scholar]

| Potential Extended Roles: |
| Anticoagulation Cardio-protective effects Cognitive function Fetal development Immune function Improved insulin sensitivity in Asians Neuronal function Reduced risk of breast cancer (women) Reduced risk of colorectal cancer (men) Reduced risk of ischemic stroke (men and women) Reduced total stroke risk (women) Retinal function Weight management |
| Potential underpinning mechanisms: |
| Altered membrane fluidity Anti-inflammatory effects Improved neurogenesis esp. in the hippocampus Modified gene expression Modified intracellular signaling Modulation of ion channels Optimized brain repair mechanisms Protected synaptic transmission Reduced production of pro-inflammatory eicosanoids |
| Life Stages (Author, Year, Location) | Population (Sample Size, Age, Gender, Health) | Study Design | Intervention (Daily Dose) | Aspect of Brain Health | Key Findings |
|---|---|---|---|---|---|
| Pregnancy/Infancy | |||||
| Brew et al. (2015) Australia [27] | n = 616 infants with a family history of asthma. | Ramdomized controlled trial (RCT) from 6 months of infancy to 5 years of age. | 500mg tuna fish oil (135 mg DHA, 32 mg EPA, 6% omega-6) per capsule or Sunola oil (control) provided after breastfeeding ceased. | Academic performance, literacy and numeracy assessment. | At 8 years, the proportion of omega-3 fatty acid in plasma was positively associated with the National Assessment Program Literacy and Numeracy score. |
| Hurtado et al. (2015) Spain [22] | n = 110 pregnant women. | RCT from 28th week of pregnancy until 4th month of lactation. | Supplemented group taking 400 mL of a fish oil-enriched dairy drink (-400 mg EPA and DHA) or 400ml control drink. | New-born visual and cognitive development. | Omega-3 LC-PUFA dietary supplement during pregnancy and lactation influenced the mother and newborn’s fatty acid profile and nervonic acid content but did not show effects on visual and cognitive/psychomotor development. |
| Sherry et al. (2015) USA [28] | n = 89 lactating women 4–6 weeks post-partum. | 6-week postpartum RCT. | 200 mg DHA, 400 mg DHA or placebo for 6 weeks with usual diets. | Maternal and infant plasma fatty acid levels. | Breast milk and maternal plasma DHA were significantly greater with 200 mg and 400 mg DHA compared with placebo which is important for brain development. |
| Gould et al. (2014) Australia [21] | n = 185 term-born children of mothers. | RCT from 20 weeks into pregnancy until measures of attention were assessed after 27 months. | 800 mg DHA or a placebo (control). | Child attention and working memory and inhibitory control. | Maternal DHA supplementation during pregnancy does not enhance attention or working memory and inhibitory control in term-born preschoolers. |
| Mulder et al. (2014) Canada [20] | n = 270 pregnant women. | RCT from week 16 of pregnancy to delivery. | 400 mg DHA or a placebo. | Central Nervous System development. | Infants in the placebo group were at increased risk of lower language development assessed as words understood and produced at 14 months and words understood and sentences produced at 18 months. |
| School age/Teenage | |||||
| Montgomery et al. (2018) UK [29] | n = 376 children aged 7–9 years underperforming in reading from primary schools in five counties of the UK. | 16-week parallel group, fixed-dose DB RCT. | 600 mg DHA (from algal oil), placebo (taste/color matched corn/soybean oil). | Reading, working memory, and behavior. | This RCT did not replicate results of the earlier DOLAB 1 study on the effectiveness of nutritional supplementation with DHA for learning and behavior. Possible reasons are discussed. |
| Arnold et al. (2017) USA [30] | n = 96 7–14 year olds Depression n = 72 Bipolar n = 23. | 12-week placebo-controlled 2X2 design. | 1.4 g EPA, 0.2 g DHA 0.27 g other n-3. | Mood symptom severity, global function. | 2 g n-3 increased EPA blood levels sevenfold and DHA levels by half (both p < 0.001). Body weight correlated inversely with increased EPA (r = −0.52, p = 0.004) and DHA (r = −0.54, p = 0.003) and positively with clinical mood response. |
| Johnson et al. (2017) Sweden [31] | n = 154 mainstream schoolchildren aged 9–10 years. | 3-month parallel, randomized, DB, placebo-controlled trial followed by 3-month active treatment for all subjects. | Three Omega 3/6 capsules (Equazen®) twice daily corresponding to a daily dose of 558 mg EPA, 174 mg DHA, and 60 mg gamma-linoleic acid) or identical placebo capsules. | Reading ability, visual analysis, phonological decoding time. | 3 months of Omega 3/6 treatment improved reading ability-specifically the clinically relevant ‘phonologic decoding time’ and ‘visual analysis time’-in mainstream schoolchildren. In particular, children with attention problems showed treatment benefits. |
| Parellada et al. (2017) Spain [32] | n = 68 children with ASD > and <12 years. | 8-week randomized, crossover, placebo-controlled study. | 962 mg for children 1155 mg for adolescents. | Changes in autistic behaviors. | Supplementation with n-3 fatty acids might be studied as an add-on to behavioral therapies in ASD. Optimal duration of treatment requires further investigation. |
| Bos et al. (2015) Netherlands [33] | n = 40 boys with ADHD, aged 8–14 years, and 39 matched, typically developing controls. | 16-week DB randomized placebo-controlled trial. | Participants consumed 10 g of margarine, enriched with either 650 mg of EPA/DHA each or placebo. | ADHD symptoms, cognitive control | Dietary supplementation with n-3 fatty acids reduced symptoms of ADHD, both for individuals with ADHD and typically developing children. |
| Milte et al. (2015) Australia [34] | n = 53 with ADHD. | 12-month randomized controlled 3-way crossover trial. | Supplements high in EPA, DHA, or linoleic acid (control) for 4 months each in a crossover design. | Attention, literacy, and behavior. | Increasing erythrocyte DHA and EPA via dietary supplementation may improve behavior, attention, and literacy in children with ADHD. |
| Portillo-Reyes et al. (2014) Spain [35] | 59 children aged 8–12 years. | 3-month randomized, DB, treatment and placebo study. | Three capsules providing n-3 (each capsule had 60 mg of DHA and 90 mg of EPA). Placebo treatment consisted of soybean oil capsules that looked similar to the active treatment. | Processing speed, attention, memory, language, executive function. | Results show that more than 50% of children in the treatment group had greater improvement in 11 of the 18 neuropsychological variables studied. Processing speed, visual-motor coordination, perceptual integration, attention and executive function showed improvement in more than 70% of the omega-3 supplemented children. |
| Widen horn-Müller et al. (2014) Germany [36] | 95 children, 6–12 years diagnosed with ADHD. | 16-week randomized, DB placebo-controlled trial. | 720 mg omega-3 fatty acids (600 mg EPA, 120 mg DHA) and 15 mg of vitamin E as antioxidant or placebo treatment. | Behavior, cognitive impairment | Supplementation with the n-3 fatty acid mix increased EPA and DHA concentrations in erythrocyte membranes and improved working memory function. Improved working memory correlated significantly with increased EPA, DHA and decreased AA. |
| Parletta et al. (2013) Australia [37] | n = 409 children aged 3–13 years. | 20-week RCT. | Omega 3/6 capsules (Equazen®) (providing 750mg DHA plus EPA, and 60mg GLA/school day) for 20 school weeks (Phase 1) followed by one-way crossover to fish oil (Phase 2). | Reading, spelling and non-verbal cognitive development | The treatment group showed improvements in Draw-A-Person compared with the placebo during Phase 1 (p = 0.029). The placebo group showed significant within-group improvements after switching to treatment (p < 0.001). |
| Young adults | |||||
| Giles et al. (2015) USA [38] | n = 72 young adults (mean age 20 years). | 35 day DB, placebo-controlled design. | 2800 mg fish oil or olive oil control. | Mood, cognition, and physiological stress. | Rated anger and confusion increased with stress in the olive oil group, but remained stable in the fish oil group. |
| Bauer et al. (2014) Australia [39] | n = 13 adults 20 to 34 years. | 30-day supplementation period. DB counterbalanced, crossover design, with a 30-day washout period between two supplementation periods. | High EPA: DHA formulation (3:1) (400 mg of natural fish oil) with added evening primrose oil (100 mg), whereas the second diet was a high DHA: EPA (4:1) formulation (365.7 mg of natural fish oil). Participants supplemented with 6 capsules daily. | Cognitive performance and functional brain activation. | Following EPA-rich supplementation, participants’ brains worked ‘less hard’ and achieved a better cognitive performance than prior to supplementation. |
| Stonehouse et al. (2013) New Zealand [40] | n = 176 healthy adults 18–45 years, non-smoking and with a low intake of DHA. | 6-month randomized, placebo-controlled, DB intervention. | 1.16 g DHA or a placebo. | Cognitive performance. | DHA supplementation improved memory and the reaction time of memory in healthy, young adults whose habitual diets were low in DHA. The response was modulated by sex. |
| Middle age | |||||
| Külzow et al. (2016) Germany [41] | n = 44 cognitively healthy individuals aged 50–75 years. | 26-week double-blind placebo-controlled proof-of-concept study. | Either LC-n3-FA (2200 mg/day, n = 22) or placebo (n = 22). | Learning and memory formation | Recall of object locations was significantly better after n3-FA supplementation compared with placebo. This study provides further experimental evidence that LC-n3-FA exert positive effects on memory functions in healthy older adults. |
| Jaremka et al. (2014) USA [42] | n = 138 (mean 51 years). | 4-month RCT. were randomized. | 1.25 g of n-3 or 2.5g of n-3. | Loneliness-related episodic memory problems. | n-3 supplementation attenuates loneliness-related verbal episodic memory declines over time and support the utility of exploring novel interventions for treating episodic memory problems among lonely people. |
| Witte et al. (2014) Germany [43] | n = 65 healthy subjects (50–75 years). | 26-week DB randomized interventional study. | Fish oil (2.2 g LC-n3-FA) or placebo. | Cognitive function | Observed a significant increase in executive functions after n3-FA compared with placebo (p = 0.023). n3-FA exerted beneficial effects on white matter microstructural integrity and gray matter volume in frontal, temporal, parietal, and limbic areas primarily of the left hemisphere. |
| Older age/Dotage | |||||
| Boespflug et al. (2016) USA [44] | n = 140 healthy adults 62–80 years with subjective memory impairment, but not meeting criteria for mild cognitive impairment or dementia. | 24-week randomized, DB, placebo-controlled study. | Fish oil (EPA + DHA: 2.4 g/day, n = 11) or placebo (corn oil, n = 10). | Cortical blood oxygen level-dependent activity during a working memory task. | Dietary fish oil supplementation increases red blood cell n-3 content, working memory performance, and blood oxygen level dependent signal in the posterior cingulate cortex during greater working memory load suggesting enhanced neuronal response to working memory challenge. |
| Bo et al. (2017) China [45] | n = 86 adults. Mean age 71 years with mild cognitive impairment. | 6-month, randomized, DB, placebo-controlled trial. | n-3 PUFAs (480 mg DHA and 720 mg EPA per day, n = 44) or placebo (olive oil, n = 42) capsules. | Cognitive function. | n-3 PUFA supplementation was associated with improved total Basic Cognitive Aptitude Test scores, perceptual speed, space imagery efficiency, and working memory (p < 0.01), but not with mental arithmetic efficiency or recognition memory (p > 0.05). |
| Hooper et al. (2017) France [46] | n = 183 ≥ 70 years reporting subjective memory complaints, but free from clinical dementia. | Secondary exploratory analysis of the above trial (Andrieu et al., 2017). | n-3 FAs (two capsules) providing a total 800 mg DHA and 225 mg EPA or placebo. | Cognitive function domains. | n-3 PUFAs may be beneficial for the maintenance of executive functioning in older adults at risk of dementia with low omega-3 index. |
| Duffy et al. (2015) Australia [47] | n = 51 older adults. Mean age 71 years. | 12-week RCT. | Four 1000-mg n-3 FA supplements (containing EPA 1200 mg plus DHA 800 mg) or placebo. | In vivo glutathione concentration. | Compared with the group given the n-3 FA supplements, the placebo group had greater change in the glutathione-to-creatine ratio in the thalamus (p = 0.049). |
| Konagai et al. (2013) Japan [48] | n = 45 healthy elderly males, 61–72 years. | 12-week randomized, DB, parallel-group comparative study. | 2 weeks of treatment with: medium-chain triglycerides as placebo; krill oil, which is rich in n-3 PUFAs incorporated in phosphatidylcholine; or sardine oil, which is abundant in n-3 PUFAs incorporated in triglycerides. | Cognitive function | n-3 PUFAs activated cognitive function in the elderly. This is especially the case with krill oil, in which the majority of n-3 PUFAs are incorporated into phosphatidylcholine, causing it to be more effective than sardine oil, in which n-3 PUFAs are present as triglycerides. |
| Publication | Randomization | Method of Randomization Described & Appropriate | Blinding Mentioned | Method of Blinding Described and Appropriate | Withdrawal and Dropout of Subjects Provided | Total Score |
|---|---|---|---|---|---|---|
| Pregnancy/Infancy | ||||||
| Brew et al. (2015) Australia [27] | 1 | 0 | 1 | 0 | 1 | 3 |
| Hurtado et al. (2015) Spain [22] | 1 | 1 | 1 | 1 | 1 | 5 |
| Sherry et al. (2015) USA [28] | 1 | 0 | 1 | 1 | 1 | 4 |
| Gould et al. (2014) Australia [21] | 1 | 1 | 1 | 0 | 1 | 4 |
| Mulder et al. (2014) Canada [20] | 1 | 1 | 1 | 1 | 1 | 5 |
| School age/Teenage | ||||||
| Montgomery et al. (2018) UK [29] | 1 | 1 | 1 | 1 | 1 | 5 |
| Arnold et al. (2017) USA [30] | 1 | 1 | 1 | 1 | 1 | 5 |
| Johnson et al. (2017) Sweden [31] | 1 | 1 | 1 | 1 | 1 | 5 |
| Parellada et al. (2017) Spain [32] | 1 | 1 | 0 | 0 | 1 | 3 |
| Bos et al. (2015) Netherlands [33] | 1 | 1 | 1 | 1 | 1 | 5 |
| Milte et al. (2015) Australia [34] | 1 | 1 | 1 | 0 | 1 | 4 |
| Portillo-Reyes et al. (2014) Spain [35] | 1 | 0 | 0 | 0 | 0 | 1 |
| Widenhorn-Müller et al. (2014) Germany [36] | 1 | 1 | 1 | 0 | 1 | 4 |
| Parletta et al. (2013) Australia [37] | 1 | 1 | 1 | 0 | 1 | 4 |
| Young adults | ||||||
| Giles et al. (2015) USA [38] | 0 | 0 | 1 | 1 | 1 | 2 |
| Bauer et al. (2014) Australia [39] | 1 | 1 | 1 | 0 | 0 | 3 |
| Stonehouse et al. (2013) [40] | 1 | 1 | 1 | 1 | 1 | 5 |
| Middle age | ||||||
| Külzow et al. (2016) Germany [41] | 1 | 1 | 1 | 1 | 1 | 5 |
| Jaremka et al. (2014) USA [42] | 1 | 0 | 1 | 1 | 0 | 3 |
| Witte et al. (2014) Germany [43] | 1 | 0 | 0 | 0 | 1 | 2 |
| Older age/Dotage | ||||||
| Boespflug et al. (2016) USA [44] | 1 | 0 | 1 | 1 | 1 | 4 |
| Bo et al. (2017) China [45] | 1 | 1 | 1 | 0 | 1 | 4 |
| Hooper et al. (2017) France [46] | 1 | 1 | 1 | 1 | 4 | 5 |
| Duffy et al. (2015) Australia [47] | 1 | 1 | 1 | 1 | 1 | 5 |
| Konagai et al. (2013) Japan [48] | 1 | 1 | 1 | 1 | 1 | 5 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094. https://doi.org/10.3390/nu10081094
Derbyshire E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients. 2018; 10(8):1094. https://doi.org/10.3390/nu10081094
Chicago/Turabian StyleDerbyshire, Emma. 2018. "Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements" Nutrients 10, no. 8: 1094. https://doi.org/10.3390/nu10081094