Consumption of Foods Derived from Subsidized Crops Remains Associated with Cardiometabolic Risk: An Update on the Evidence Using the National Health and Nutrition Examination Survey 2009–2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Subsidy Score
2.3. Cardiometabolic Risk Measures
2.4. Covariates
2.5. Statistical Analysis
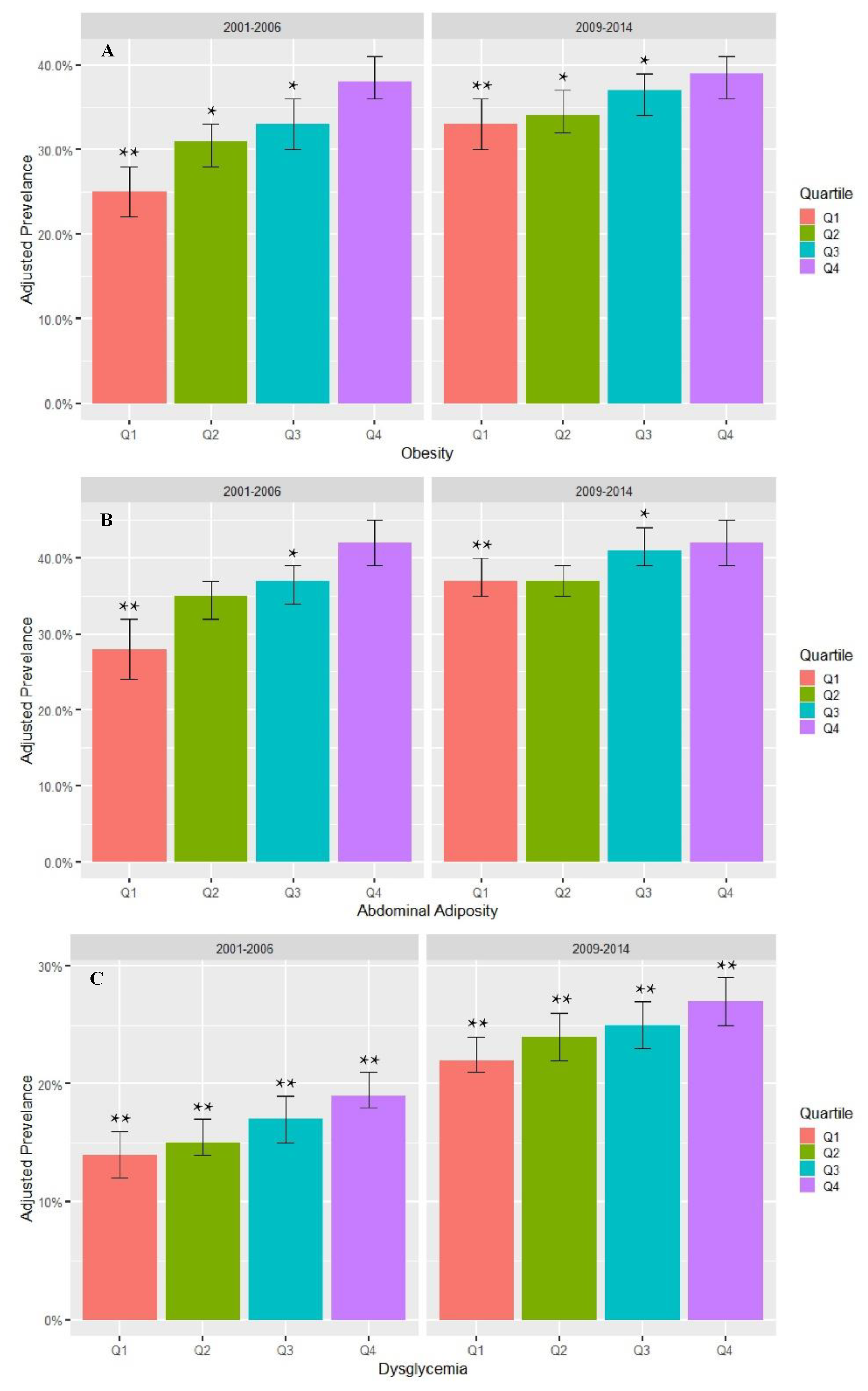
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Hales, C.; Carroll, M.; Fryar, C.; Ogden, C. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief n. 360; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Narayan, K.M.V.; Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Williamson, D.F. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007, 30, 1562–1566. [Google Scholar] [CrossRef] [Green Version]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Griffin, T.; Mande, J. The 2018 farm bill—Implications and opportunities for public health. JAMA 2019, 321, 835–836. [Google Scholar] [CrossRef]
- Corn & Other Feedgrains. Available online: https://www.ers.usda.gov/topics/crops/corn-and-other-feedgrains/ (accessed on 9 September 2020).
- Monteiro, C.A.; Moubarac, J.-C.; Levy, R.B.; Canella, D.S.; Louzada, M.L.D.C.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. Br. Med. J. 2015, 351, h3576. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 12, 2271–2283. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.S.; Del-Ponte, B.; Assuncao, M.C.F.; Santos, I.S. Consumption of ultra-processed foods and body fat during childhood and adolescence: A systematic review. Public Health Nutr. 2018, 21, 148–159. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Taxes on Sugary Drinks: Why do it? World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Peñalvo, J.L.; Cudhea, F.; Micha, R.; Rehm, C.D.; Afshin, A.; Whitsel, L.; Wilde, P.; Gaziano, T.; Pearson-Stuttard, J.; O’Flaherty, M.; et al. The potential impact of food taxes and subsidies on cardiovascular disease and diabetes burden and disparities in the United States. BMC Med. 2017, 15, 208. [Google Scholar] [CrossRef] [Green Version]
- Siegel, K.R.; McKeever Bullard, K.; Imperatore, G.; Kahn, H.S.; Stein, A.D.; Ali, M.K.; Narayan, K.M. Association of higher consumption of foods derived from subsidized commodities with adverse cardiometabolic risk among US adults. JAMA Intern. Med. 2016, 176, 1124–1132. [Google Scholar] [CrossRef]
- Siegel, K.R.; McKeever Bullard, K.; Ali, M.K.; Stein, A.D.; Kahn, H.S.; Mehta, N.K.; Webb Girard, A.; Narayan, K.M.; Imperatore, G. The contribution of subsidized food commodities to total energy intake among US adults. Public Health Nutr. 2016, 19, 1348–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chite, R.M. Farm Bill Budget and Costs: 2002 vs. 2007; Congressional Research Service: Washington, DC, USA, 2008.
- Macy, J.T.; Chassin, L.; Presson, C.C. Predictors of health behaviors after the economic downturn: A longitudinal study. Soc. Sci. Med. 2013, 89, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.W.; Slining, M.M.; Popkin, B.M. Turning point for US diets? Recessionary effects or behavioral shifts in foods purchased and consumed. Am. J. Clin. Nutr. 2014, 99, 609–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margerison-Zilko, C.; Goldman-Mellor, S.; Falconi, A.; Downing, J. Health impacts of the great recession: A critical review. Curr. Epidemiol. Rep. 2016, 3, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Monke, J.; Assenberg, R.A.; Stubbs, M. Expiration and Extension of the 2008 Farm Bill; Congressional Research Service: Washington, DC, USA, 2013.
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES dietary data: Focus on collection, release, analytical considerations, and uses to inform public policy. Adv. Nutr. (Bethesda Md) 2016, 7, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data 2009–2010; Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2011.
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data 2011–2012; Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2013.
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data 2013–2014; Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2015.
- What We Eat in America, NHANES 2009–2010; U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group: Beltsville, MD, USA; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2011.
- What We Eat in America, NHANES 2011–2012; U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group: Beltsville, MD, USA; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2013.
- What We Eat in America, NHANES 2013–2014; U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group: Beltsville, MD, USA; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2015.
- Food Patterns Equivalents Database 2009–2010; Food Survey Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2013.
- Food Patterns Equivalents Database 2011–2012; Food Survey Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2014.
- Food Patterns Equivalents Database 2013–2014; Food Survey Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2017.
- Food Intakes Converted to Retail Commodities Databases 2007–2008; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2013.
- US Department of Agriculture ARS, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28; US Department of Agriculture ARS, Nutrient Data Laboratory: Beltsville, MD, USA, 2016.
- National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. 2013. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Anthropometry.pdf (accessed on 9 September 2020).
- National Health and Nutrition Examination Survey (NHANES) MEC Laboratory Procedure Manual. 2013. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/2013_MEC_Laboratory_Procedures_Manual.pdf (accessed on 9 September 2020).
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Lu, Y.; Qi, H.; Li, F.; Shen, Z.; Wu, L.; Yang, C.; Wang, L.; Shui, K.; Yao, W.; et al. Waist-to-height ratio is an effective indicator for comprehensive cardiovascular health. Sci. Rep. 2017, 7, 43046. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 41 (Suppl. 1), S13–S28. [Google Scholar]
- Rehm, C.D.; Peñalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary intake among US adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef] [PubMed]
- Gerlt, S.; Thompson, W.; Johansson, R.; Syndow, S. Now that it’s 2016, let’s compare 2014 farm bill programs to the 2008 farm bill. Farmdoc Dly. 2016, 6, 128. [Google Scholar]
- Camilleri, G.M.; Méjean, C.; Kesse-Guyot, E.; Andreeva, V.A.; Bellisle, F.; Hercberg, S.; Péneau, S. The associations between emotional eating and consumption of energy-dense snack foods are modified by sex and depressive symptomatology. J. Nutr. 2014, 144, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Batty, G.D.; Pentti, J.; Shipley, M.J.; Sipilä, P.N.; Nyberg, S.T.; Suominen, S.B.; Oksanen, T.; Stenholm, S.; Virtanen, M.; et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: A multi-cohort study. Lancet Public Health 2020, 5, e140–e149. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Leung, C.W.; Li, Y.; Ding, E.L.; Chiuve, S.E.; Hu, F.B.; Willett, W.C. Trends in dietary quality among adults in the United States, 1999 Through 2010. JAMA Intern. Med. 2014, 174, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Office of the First Lady: First Lady Michelle Obama Launches Let’s Move: America’s Move to Raise a Healthier Generation of Kids; The White House: Washington, DC, USA, 2010.
- Holzman, D.C. White House proposes healthy food financing initiative. Environ. Health Perspect. 2010, 118, A156. [Google Scholar] [CrossRef]
- Yang, L.; Cao, C.; Kantor, E.D.; Nguyen, L.H.; Zheng, X.; Park, Y.; Giovannucci, E.L.; Matthews, C.E.; Colditz, G.A.; Cao, Y. Trends in sedentary behavior among the US population, 2001–2016. JAMA 2019, 321, 1587–1597. [Google Scholar] [CrossRef]
- Krueger, P.M.; Reither, E.N. Mind the gap: Race/ethnic and socioeconomic disparities in obesity. Curr. Diab. Rep. 2015, 15, 95. [Google Scholar] [CrossRef] [Green Version]
- Franck, C.; Grandi, S.M.; Eisenberg, M.J. Agricultural subsidies and the American obesity epidemic. Am. J. Prev. Med. 2013, 45, 327–333. [Google Scholar] [CrossRef]
- Food Availability (Per Capita) Data System; USDA Economic Research Service: Washington, DC, USA, 2019.
- Siegel, K.R.; Ali, M.K.; Srinivasiah, A.; Nugent, R.A.; Narayan, K.M. Do we produce enough fruits and vegetables to meet global health need? PLoS ONE 2014, 9, e104059. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ (Clin. Res. Ed.) 2018, 361, k2396. [Google Scholar] [CrossRef] [Green Version]
- Cobiac, L.J.; Tam, K.; Veerman, L.; Blakely, T. Taxes and subsidies for improving diet and population health in Australia: A cost-effectiveness modelling study. PLoS Med. 2017, 14, e1002232. [Google Scholar] [CrossRef]
- Blakely, T.; Cleghorn, C.; Mizdrak, A.; Waterlander, W.; Nghiem, N.; Swinburn, B.; Wilson, N.; Ni Mhurchu, C. The effect of food taxes and subsidies on population health and health costs: A modelling study. Lancet Public Health 2020, 5, e404–e413. [Google Scholar] [CrossRef]
- Grandjean, A.C. Dietary intake data collection: Challenges and limitations. Nutr. Rev. 2012, 70 (Suppl. 2), S101–S104. [Google Scholar] [CrossRef]
- Congressional Research Service: U.S. Food and Agricultural Imports: Safeguards and Selected Issues. 2020. Available online: https://crsreports.congress.gov/product/pdf/R/R46440 (accessed on 9 September 2020).


| Variable | Unweighted Number | Weighted Distribution A | Q1 (0.00–0.41) A | Q2 (0.42–0.53) A | Q3 (0.54–0.63) A | Q4 (0.64–1.00) A | p-Value B |
|---|---|---|---|---|---|---|---|
| Subsidy Score, mean (95% CI) | 12,039 | 0.50 (0.50–0.51) | 0.30 (0.30–0.31) | 0.47 (0.47–0.47) | 0.58 (0.58–0.59) | 0.72 (0.71–0.72) | |
| Male | 6279 | 49.5 (0.5) | 48.6 (0.9) | 50.3 (1.0) | 48.1 (1.4) | 51.0 (1.1) | 0.97 |
| Age group, year | |||||||
| 18–24 | 2058 | 15.2 (0.8) | 13.3 (1.1) | 13.6 (1.0) | 15.8 (1.6) | 18.7 (1.0) | <0.0001 |
| 25–34 | 2360 | 20.7 (0.6) | 19.6 (1.1) | 21.3 (1.0) | 20.4 (1.2) | 21.8 (1.0) | |
| 35–44 | 2445 | 21.0 (0.7) | 21.5 (1.1) | 20.3 (1.0) | 21.4 (1.1) | 21.0 (1.2) | |
| 45–54 | 2498 | 22.8 (0.7) | 24.0 (1.1) | 22.7 (1.0) | 23.5 (1.6) | 20.6 (1.0) | |
| 55–64 | 2403 | 20.3 (0.6) | 21.7 (1.1) | 22.1 (1.1) | 18.9 (1.3) | 17.8 (1.0) | |
| Age, mean (SE), year | 12,039 | 40.9 (0.3) | 41.9 (0.4) | 41.6 (0.4) | 40.6 (0.6) | 39.4 (0.4) | <0.0001 |
| Race/ethnicity | |||||||
| Non-Hispanic white | 4683 | 65.1 (2.1) | 63.9 (2.5) | 67.2 (2.3) | 65.6 (2.5) | 63.9 (2.6) | 0.41 |
| Non-Hispanic black | 2549 | 11.5 (1.0) | 11.8 (1.3) | 11.3 (1.2) | 11.2 (1.1) | 12.0 (1.0) | |
| Mexican American | 1884 | 9.6 (1.2) | 8.9 (1.3) | 9.0 (1.0) | 9.8 (1.4) | 10.8 (1.7) | |
| Other | 2648 | 13.8 (0.8) | 15.4 (1.2) | 12.6 (1.0) | 13.3 (1.2) | 13.5 (1.3) | |
| Education Attainment | |||||||
| <High school graduate | 2716 | 15.9 (0.8) | 12.8 (1.1) | 15.2 (1.2) | 16.1 (1.1) | 20.5 (1.1) | <0.0001 |
| High school graduate | 2735 | 21.6 (0.7) | 19.4 (1.2) | 19.4 (0.8) | 24.3 (1.5) | 24.4 (1.2) | |
| Some college | 3737 | 32.2 (0.8) | 33.1 (1.3) | 31.0 (1.2) | 31.6 (1.5) | 33.1 (1.1) | |
| ≥College graduate | 2839 | 30.3 (1.2) | 34.7 (1.8) | 34.5 (1.3) | 28.1 (1.8) | 22.0 (1.5) | |
| Poverty Income Ratio, % C | |||||||
| <130 | 3883 | 24.1 (1.2) | 21.0 (1.5) | 22.4 (1.4) | 25.0 (1.6) | 29.2 (1.6) | <0.0001 |
| 130 to <185 | 1311 | 9.9 (0.5) | 9.0 (0.7) | 10.0 (0.7) | 10.0 (0.8) | 11.1 (0.9) | |
| ≥185 | 5647 | 65.9 (1.4) | 70.0 (1.7) | 67.6 (1.6) | 65.0 (1.6) | 59.7 (2.0) | |
| Smoking status | |||||||
| Current | 2648 | 22.0 (0.7) | 20.8 (1.0) | 21.1 (1.0) | 22.7 (1.3) | 24.2 (1.5) | 0.70 |
| Past | 2074 | 20.3 (0.8) | 22.7 (1.0) | 20.4 (1.0) | 19.1 (1.5) | 18.2 (1.1) | |
| Never | 6524 | 57.6 (1.0) | 56.5 (1.1) | 58.5 (1.3) | 58.2 (2.1) | 57.6 (1.7) | |
| Daily energy, mean (SE), kcal | 12,039 | 2233.05 (9.8) | 2257.8 (20.0) | 2265.8 (20.1) | 2240.0 (25.1) | 2159.6 (19.3) | 0.0004 |
| Leisure-time physical activity D | |||||||
| Yes | 6223 | 57.3 (1.0) | 62.1 (1.4) | 57.6 (1.5) | 56.9 (1.3) | 51.2 (1.3) | <0.0001 |
| No | 5539 | 42.7 (1.0) | 37.9 (1.4) | 42.4 (1.5) | 43.1 (1.3) | 48.8 (1.3) |
| Variable | Overall Mean A | Q1 (0.00–0.41) A | Q2 (0.42–0.53) A | Q3 (0.54–0.63) A | Q4 (0.64–1.00) A | p-Value B |
|---|---|---|---|---|---|---|
| Body mass index (kg/m2) | 28.8 (28.5, 29.0) | 28.5 (28.1, 28.9) | 28.8 (28.5, 29.1) | 29.0 (28.6, 29.4) | 29.4 (29.0, 29.8) | 0.003 |
| Ratio of waist circumference to height | 0.578 (0.574–0.582) | 0.575 (0.569–0.580) | 0.576 (0.571–0.580) | 0.584 (0.578–0.590) | 0.586 (0.581–0.592) | <0.0001 |
| Systolic blood pressure, mm Hg | 118.7 (118.2–119.2) | 118.8 (118.0–119.6) | 118.9 (118.3–119.5) | 119.1 (118.2–120.0) | 118.8 (117.9–119.6) | 0.90 |
| Diastolic blood pressure, mm Hg | 71.2 (70.6–71.8) | 71.2 (70.6–71.8) | 71.4 (70.7–71.1) | 71.5 (70.7–72.2) | 71.9 (70.9–72.9) | 0.60 |
| Non-HDL cholesterol concentration, mg/dL | 140.7 (139.4, 142.0) | 139.4 (137.8, 140.9) | 140.8 (138.6, 142.9) | 141.8 (139.8, 143.7) | 143.8 (141.6, 146.1) | 0.004 |
| Hemoglobin A1c level, % | 5.54 (5.52–5.56) | 5.51 (5.47, 5.54) | 5.54 (5.50, 5.58) | 5.56 (5.52, 5.60) | 5.58 (5.54, 5.62) | 0.037 |
| Variable | Coefficient (Standard Error) A | p-Value B |
|---|---|---|
| Body mass index (kg/m2) | 0.23 (0.05) | <0.0001 |
| Ratio of waist circumference to height | 0.003 (0.001) | <0.0001 |
| Systolic blood pressure, mm Hg | 0.05 (0.001) | 0.68 |
| Diastolic blood pressure, mm Hg | 0.17 (0.11) | 0.11 |
| Non-HDL cholesterol concentration, mg/dL | 1.11 (0.26) | <0.0001 |
| Hemoglobin A1c level, % | 0.02 (0.006) | <0.0001 |
| Food Component | Mean (SE) 2001–2006 | Mean (SE) 2009–2014 | Relative Change | p-Value |
|---|---|---|---|---|
| Corn Sweetener | 0.085 (0.002) | 0.075 (0.001) | 12% | <0.0001 |
| Dairy | 0.117 (0.002) | 0.100 (0.001) | 14% | <0.0001 |
| Grains | 0.223 (0.001) | 0.201 (0.001) | 10% | <0.0001 |
| Soy | 0.048 (0.005) | 0.042 (0.004) | 14% | 0.002 |
| Eggs | 0.015 (0.0004) | 0.008 (0.0002) | 51% | <0.0001 |
| Meat | 0.133 (0.001) | 0.129 (0.002) | 2% | 0.21 |
| Corn-fed Fish | 0.00006 (0.00005) | 0.00005 (0.0004) | 32% | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, W.L.; Bullard, K.M.; Stein, A.D.; Ali, M.K.; Narayan, K.M.V.; Siegel, K.R. Consumption of Foods Derived from Subsidized Crops Remains Associated with Cardiometabolic Risk: An Update on the Evidence Using the National Health and Nutrition Examination Survey 2009–2014. Nutrients 2020, 12, 3244. https://doi.org/10.3390/nu12113244
Do WL, Bullard KM, Stein AD, Ali MK, Narayan KMV, Siegel KR. Consumption of Foods Derived from Subsidized Crops Remains Associated with Cardiometabolic Risk: An Update on the Evidence Using the National Health and Nutrition Examination Survey 2009–2014. Nutrients. 2020; 12(11):3244. https://doi.org/10.3390/nu12113244
Chicago/Turabian StyleDo, Whitney L., Kai M. Bullard, Aryeh D. Stein, Mohammed K. Ali, K. M. Venkat Narayan, and Karen R. Siegel. 2020. "Consumption of Foods Derived from Subsidized Crops Remains Associated with Cardiometabolic Risk: An Update on the Evidence Using the National Health and Nutrition Examination Survey 2009–2014" Nutrients 12, no. 11: 3244. https://doi.org/10.3390/nu12113244





