Epidemiologic Studies of Isoflavones & Mammographic Density
Abstract
:1. Introduction
2. Background
2.1. Isoflavones
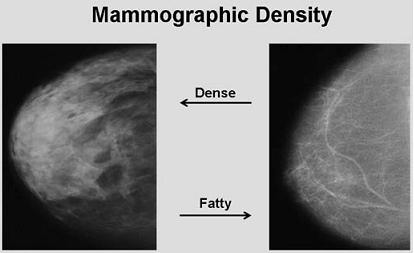
2.2. Mammographic Density and Breast Cancer Risk
2.3. Steroid Hormones and Mammographic Density
3. Results
3.1. Observational Studies
| Author, Year | N Status | Isoflavone intake (mg) | Assessment method3 | Results for lowest and highest intake categories | ||
|---|---|---|---|---|---|---|
| Maskarinec, 2001 [51] | 514 Pre & post | Asians | 81 | Computer-assisted | Asians | 38.2% and 34.5%; p = 0.31 |
| Whites | 41 | Whites | 26.7% and 30.7%; p = 0.06 | |||
| Nagel, 2005 [52] | 54 Pre & post | 0.1 | Wolfe categories | 0.01 vs. 0.19 mg isoflavones in women with dense vs. non-dense breast; p = 0.26 | ||
| Nagata, 2005 [55] | 601 Pre & post | Pre | 42 | Automated | Pre | 30.2% and 37.8%; p = 0.28 |
| Post | 75 | Post | 9.7% and 13.1%; p = 0.33 | |||
| Jakes, 2002 [53]2 | 406 Pre & post | 14 | Tabar categories | Odds ratio of 1 and 0.44 to have dense breasts; p = 0.07 | ||
| Ursin, 2006 [54]2 | 380 Pre & post | 15 | Computer-assisted | 26.1% and 21.2%; p = 0.03 | ||
| Wu, 2008 [56] | 3,315 Pre & post | Pre | 17 | Computer-assisted | 25.7% and 26.3%; p = 0.28 | |
| Post | 18 | |||||
3.2. Randomized Trials
| Author Year | N Status | Treatment | Iso dose (mg) | Duration (years) | Assessment method4 | Change in density (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Intervention | ||||||||
| Maskarinec 2003 [58] | 30 Pre | Isoflavone supplement | 100 | 1 | Computer-assisted | 0.4 | 2.5 | ||
| Maskarinec 2004 [59] | 213 Pre | Soy foods | 50 | 2 | Computer-assisted | -4.1 | -2.8 | ||
| Powles 2008 [64] | 401 Pre & post | Red clover supplement | 40 | 3 | Visual estimation | Pre | -3.0 | Pre | -6.6 |
| Post | -8.0 | Post | -6.9 | ||||||
| Verheus 2008 [63] | 126 Post | Soy powder | 99 | 1 | Computer-assisted | -4.6 | -2.5 | ||
| Marini 2008 [61] | 138 Post | Genistein supplement | 54 | 3 | Wolfe categories | 13.4 1 | 22.5 1 | ||
| Computer-assisted | -0.4 2 | -0.4 2 | |||||||
| Atkinson 2004 [60]3 | 177 Pre & post | Red clover supplement | 45 | 1 | Visual estimation | -3.9 | -3.2 | ||
| Kataoka 2008 [65]3 | 177 Pre & post | Red clover supplement | 45 | 1 | Computer-assisted | -0.5 | -2.1 | ||
| Volumetric | 0.8 | 0.3 | |||||||
| Maskarinec 2009 [62] | 358 Post | Isoflavone supplement | 80 & 120 | 2 | Computer-assisted | -1.4/y | 80 mg | -1.6/y | |
| 120 mg | -1.3/y | ||||||||
3.3. Equol-Producer Status and Breast Density
4. Discussion and Conclusions
References
- Trock, B.J.; Hilakivi-Clarke, L.; Clarke, R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 459–471. [Google Scholar]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14. [Google Scholar]
- Shu, X.O.; Jin, F.; Dai, Q.; Wen, W.; Potter, J.D.; Kushi, L.H.; Ruan, Z.; Gao, Y.T.; Zheng, W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 483–488. [Google Scholar]
- Wu, A.H.; Wan, P.; Hankin, J.; Tseng, C.C.; Yu, M.C.; Pike, M.C. Adolescent and adult soy intake and risk of breast cancer in Asian- Americans. Carcinogenesis 2002, 23, 1491–1496. [Google Scholar]
- United States Department of Agriculture USDA-Iowa State University Database on the Isoflavone Content of Foods. 2002. Available online: http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/ isoflav.html/ accessed on January 11, 2010.
- Adlercreutz, H.; Goldin, B.R.; Gorbach, S.L.; Höckerstedt, K.A.V.; Watanabe, S.; Hämäläinen, E.; Markkanen, M.H.; Mäkelä, T.K.; Wähäläm, K.T.; Hase, T.A.; Fotsis, T. Soybean phytoestrogen intake and cancer risk. J. Nutr. 1995, 125, 757S–770S. [Google Scholar]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar]
- Tsunoda, N.; Pomeroy, S.; Nestel, P. Absorption in humans of isoflavones from soy and red clover is similar. J. Nutr. 2002, 132, 2199–2201. [Google Scholar]
- McCarty, M.F. Isoflavones made simple - genistein's agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypotheses 2006, 66, 1093–1114. [Google Scholar]
- Gallo, D.; Ferrandina, G.; Giacomelli, S.; Fruscella, E.; Zannoni, G.; Morazzoni, P.; Riva, A.; Bombardelli, E.; Mancuso, S.; Scambia, G. Dietary soy modulation of biochemical parameters in DMBA-induced mammary tumors. Cancer Lett. 2002, 186, 43–48. [Google Scholar]
- Lazennec, G.; Bresson, D.; Lucas, A.; Chauveau, C.; Vignon, F. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology 2001, 142, 4120–30. [Google Scholar]
- Fox, E.M.; Davis, R.J.; Shupnik, M.A. ERbeta in breast cancer--onlooker, passive player, or active protector? Steroids 2008, 73, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Field, A.S.; Tran, D.D.; Killeen, J.; Maskarinec, G.; Ishikura, H.; Trichopoulos, D. Breast cancer incidence and estrogen receptor alpha in normal mammary tissue--an epidemiologic study among Japanese women in Japan and Hawaii. Int. J. Cancer 2002, 97, 685–687. [Google Scholar]
- Messina, M.J.; Persky, V.; Setchell, K.D.; Barnes, S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer 1994, 21, 113–131. [Google Scholar]
- Messina, M.J.; Loprinzi, C.L. Soy for breast cancer survivors: a critical review of the literature. J. Nutr. 2001, 131, 3095S–3108S. [Google Scholar]
- Loukovaara, M.; Carson, M.; Palotie, A.; Adlercreutz, H. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids 1995, 60, 656–661. [Google Scholar]
- Adlercreutz, H.; Bannwart, C.; Wahala, K.; Makela, T.; Brunow, G.; Hase, T.; Arosemena, P.J.; Kellis, J.; Vickery, L.E. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993, 44, 147–153. [Google Scholar]
- Makela, S.; Poutanen, M.; Lehtimaki, J.; Kostian, M.L.; Santti, R.; Vihko, R. Estrogen-specific 17β-hydroxysteroid oxidoreductase type I (E.C.1.1.1.62) as a possible target for the action of phytoestrogens. Proc. Soc. Exp. Biol. Med. 1995, 208, 51–59. [Google Scholar] [PubMed]
- Rose, D.P. Dietary fiber and breast cancer. Nutr. Cancer 1990, 13, 1–8. [Google Scholar]
- Adlercreutz, H. Evolution, nutrition, intestinal microflora, and prevention of cancer: a hypothes. Proc. Soc. Exp. Biol. Med. 1998, 217, 241–246. [Google Scholar]
- Barnes, S.; Boersma, B.; Patel, R.; Kirk, M.; Darley-Usmar, V.M.; Kim, H.; Xu, J. Isoflavonoids and chronic disease: mechanisms of action. Biofactors 2000, 12, 209–215. [Google Scholar]
- Ford, D. Mechanistic explanations for the chemopreventive action of soyabean isoflavones: reducing the possibilities. Br. J. Nutr. 2002, 88, 439–441. [Google Scholar]
- Cotroneo, M.S.; Wang, J.; Fritz, W.A.; Eltoum, I.E.; Lamartiniere, C.A. Genistein action in the prepubertal mammary gland in a chemoprevention model. Carcinogenesis 2002, 23, 1467–1474. [Google Scholar]
- Russo, J.; Russo, I.H. Hormonally induced differentiation: a novel approach to breast cancer prevention. J. Cell. Biochem. Suppl. 1995, 22, 58–64. [Google Scholar]
- Hilakivi-Clarke, L.; Onojafe, I.; Raygada, M.; Cho, E.; Skaar, T.; Russo, I.; Clarke, R. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br. J. Cancer 1999, 80, 1682–1688. [Google Scholar]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006, 15, 1159–1169. [Google Scholar]
- Wolfe, J.N. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 1976, 37, 2486–2492. [Google Scholar]
- Gram, I.T.; Funkhouser, E.; Tabar, L. Reproductive and menstrual factors in relation to mammographic parenchymal patterns among perimenopausal women. Br. J. Cancer 1995, 71, 647–650. [Google Scholar]
- Saftlas, A.F.; Hoover, R.N.; Brinton, L.A.; Szklo, M.; Olson, D.R.; Salane, M.; Wolfe, J.N. Mammographic densities and risk of breast cancer. Cancer 1991, 67, 2833–2838. [Google Scholar]
- Byrne, C.; Schairer, C.; Wolfe, J.; Parekh, N.; Salane, M.; Brinton, L.A.; Hoover, R.; Haile, R. Mammographic features and breast cancer risk: effects with time, age, and menopause statu. J. Natl. Cancer Inst. 1995, 87, 1622–1629. [Google Scholar]
- Byng, J.W.; Boyd, N.F.; Fishell, E.; Jong, R.A.; Yaffe, M.J. The quantitative analysis of mammographic densities. Phys. Med. Biol. 1994, 39, 1629–1638. [Google Scholar]
- Ursin, G.; Astrahan, M.A.; Salane, M.; Parisky, Y.R.; Pearce, J.G.; Daniels, J.R.; Pike, M.C.; Spicer, D.V. The detection of changes in mammographic densities. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 43–47. [Google Scholar]
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A.D. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar]
- Gram, I.T.; Bremnes, Y.; Ursin, G.; Maskarinec, G.; Bjurstam, N.; Lund, E. Percentage density, Wolfe's and Tabar's mammographic patterns: agreement and association with risk factors for breast cancer. Breast Cancer Res. 2005, 7, R854–R861. [Google Scholar]
- Boyd, N.; Martin, L.; Gunasekara, A.; Melnichouk, O.; Maudsley, G.; Peressotti, C.; Yaffe, M.; Minkin, S. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1754–1762. [Google Scholar]
- Highnam, R.; Pan, X.; Warren, R.; Jeffreys, M.; Davey, S.G.; Brady, M. Breast composition measurements using retrospective standard mammogram form (SMF). Phys. Med. Biol. 2006, 51, 2695–2713. [Google Scholar]
- Martin, L.J.; Minkin, S.; Boyd, N.F. Hormone therapy, mammographic density, and breast cancer ris. Maturitas 2009, 64, 20–26. [Google Scholar]
- Greendale, G.A.; Reboussin, B.A.; Slone, S.; Wasilauskas, C.; Pike, M.C.; Ursin, G. Postmenopausal hormone therapy and change in mammographic density. J. Natl. Cancer Inst. 2003, 95, 30–37. [Google Scholar]
- McTiernan, A.; Martin, C.F.; Peck, J.D.; Aragaki, A.K.; Chlebowski, R.T.; Pisano, E.D.; Wang, C.Y.; Brunner, R.L.; Johnson, K.C.; Manson, J.E.; Lewis, C.E.; Kotchen, J.M.; Hulka, B.S. Estrogen-plus-progestin use and mammographic density in postmenopausal women: women's health initiative randomized trial. J. Natl. Cancer Inst. 2005, 97, 1366–1376. [Google Scholar]
- Pike, M.C. The role of mammographic density in evaluating changes in breast cancer risk. Gynecol. Endocrinol. 2005, 21 Suppl 1, 1–5. [Google Scholar]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; Kotchen, J.M.; Ockene, J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Spicer, D.V.; Ursin, G.; Parisky, Y.R.; Pearce, J.G.; Shoupe, D.; Pike, A.; Pike, M.C. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J. Natl. Cancer Inst. 1994, 86, 431–436. [Google Scholar]
- Cuzick, J.; Warwick, J.; Pinney, E.; Warren, R.M.; Duffy, S.W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Natl. Cancer Inst. 2004, 96, 621–628. [Google Scholar]
- Freedman, M.; San Martin, J.; O'Gorman, J.; Eckert, S.; Lippman, M.E.; Lo, S.C.; Walls, E.L.; Zeng, J. Digitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placeb. J. Natl. Cancer Inst. 2001, 93, 51–56. [Google Scholar]
- Becker, S.; Kaaks, R. Exogenous and endogenous hormones, mammographic density and breast cancer risk: can mammographic density be considered an intermediate marker of risk? Recent Results Cancer Res 2009, 181, 135–157. [Google Scholar]
- Greendale, G.A.; Palla, S.L.; Ursin, G.; Laughlin, G.A.; Crandall, C.; Pike, M.C.; Reboussin, B.A. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am. J. Epidemiol. 2005, 162, 826–834. [Google Scholar]
- Boyd, N.F.; Stone, J.; Martin, L.J.; Jong, R.; Fishell, E.; Yaffe, M.; Hammond, G.; Minkin, S. The association of breast mitogens with mammographic densities. Br. J. Cancer 2002, 87, 876–882. [Google Scholar]
- Tamimi, R.M.; Byrne, C.; Colditz, G.A.; Hankinson, S.E. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2007, 99, 1178–1187. [Google Scholar]
- Verheus, M.; Peeters, P.H.; Van Noord, P.A.; van der Schouw, Y.T.; Grobbee, D.E.; van Gils, C.H. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast Cancer Res. 2007, 9, R53. [Google Scholar]
- Noh, J.J.; Maskarinec, G.; Pagano, I.; Cheung, L.W.; Stanczyk, F.Z. Mammographic densities and circulating hormones: a cross-sectional study in premenopausal women. Breast 2006, 15, 20–28. [Google Scholar]
- Maskarinec, G.; Meng, L. An investigation of soy intake and mammographic characteristics in Hawaii. Breast Cancer Res. 2001, 3, 134–141. [Google Scholar]
- Nagel, G.; Mack, U.; von, F.D.; Linseisen, J. Dietary phytoestrogen intake and mammographic density -- results of a pilot study. Eur. J. Med. Res. 2005, 10, 389–394. [Google Scholar]
- Jakes, R.W.; Duffy, S.W.; Ng, F.C.; Gao, F.; Ng, E.H.; Seow, A.; Lee, H.P.; Yu, M.C. Mammographic parenchymal patterns and self-reported soy intake in Singapore Chinese women. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 608–613. [Google Scholar]
- Ursin, G.; Sun, C.L.; Koh, W.P.; Khoo, K.S.; Gao, F.; Wu, A.H.; Yu, M.C. Associations between soy, diet, reproductive factors, and mammographic density in Singapore Chinese women. Nutr. Cancer 2006, 56, 128–135. [Google Scholar]
- Nagata, C.; Matsubara, T.; Fujita, H.; Nagao, Y.; Shibuya, C.; Kashiki, Y.; Shimizu, H. Associations of mammographic density with dietary factors in Japanese women. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 2877–2880. [Google Scholar]
- Wu, A.H.; Ursin, G.; Koh, W.P.; Wang, R.; Yuan, J.M.; Khoo, K.S.; Yu, M.C. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3358–3365. [Google Scholar]
- Maskarinec, G.; Pagano, I.; Lurie, G.; Kolonel, L.N. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 732–739. [Google Scholar]
- Maskarinec, G.; Williams, A.E.; Carlin, L. Mammographic densities in a one-year isoflavone intervention. Eur. J. Cancer Prev. 2003, 12, 165–169. [Google Scholar]
- Maskarinec, G.; Takata, Y. ; Franke, A.A.; Williams, A.E.; Murphy, S.P. A 2-year soy intervention in premenopausal women does not change mammographic densities. J. Nutr. 2004, 134, 3089–3094. [Google Scholar] [PubMed]
- Atkinson, C.; Warren, R.M.; Sala, E.; Dowsett, M.; Dunning, A.M.; Healey, C.S.; Runswick, S.; Day, N.E.; Bingham, S.A. Red clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004, 6, R170–R179. [Google Scholar]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di, S.V; Minutoli, L.; Atteritano, M.; Levy, R.M.; D'Anna, R.; Frisina, N.; Mazzaferro, S.; Cancellieri, F.; Cannata, M.L.; Corrado, F.; Frisina, A.; Adamo, V.; Lubrano, C.; Sansotta, C.; Marini, R.; Adamo, E.B.; Squadrito, F. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J. Clin. Endocrinol. Metab 2008, 93, 4787–4796. [Google Scholar] [PubMed]
- Maskarinec, G.; Verheus, M.; Steinberg, F.M.; Amato, P.; Cramer, M.K.; Lewis, R.D.; Murray, M.J.; Young, R.L.; Wong, W.W. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J. Nutr. 2009, 139, 981–986. [Google Scholar]
- Verheus, M.; van Gils, C.H.; Kreijkamp-Kaspers, S.; Kok, L.; Peeters, P.H.; Grobbee, D.E.; van der Schouw, Y.T. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2632–2638. [Google Scholar]
- Powles, T.J.; Howell, A.; Evans, D.G.; McCloskey, E.V.; Ashley, S.; Greenhalgh, R.; Affen, J.; Flook, L.A.; Tidy, A. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause. Int. 2008, 14, 6–12. [Google Scholar]
- Kataoka, M.; Atkinson, C.; Warren, R.; Sala, E.; Day, N.E.; Highnam, R.; Warsi, I.; Bingham, S.A. Mammographic density using two computer-based methods in an isoflavone trial. Maturitas 2008, 59, 350–357. [Google Scholar]
- Vachon, C.M.; Pankratz, V.S.; Scott, C.G.; Maloney, S.D.; Ghosh, K.; Brandt, K.R.; Milanese, T.; Carston, M.J.; Sellers, T.A. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 921–928. [Google Scholar]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar]
- Frankenfeld, C.L.; McTiernan, A.; Aiello, E.J.; Thomas, W.K.; LaCroix, K.; Schramm, J.; Schwartz, S.M.; Holt, V.L.; Lampe, J.W. Mammographic density in relation to daidzein-metabolizing phenotypes in overweight, postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1156–1162. [Google Scholar]
- Fuhrman, B.J.; Teter, B.E.; Barba, M.; Byrne, C.; Cavalleri, A.; Grant, B.J.; Horvath, P.J.; Morelli, D.; Venturelli, E.; Muti, P.C. Equol status modifies the association of soy intake and mammographic density in a sample of postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 33–42. [Google Scholar]
- Tamimi, R.M.; Hankinson, S.E.; Colditz, G.A.; Byrne, C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 2641–2647. [Google Scholar]
- Boyd, N.F.; Martin, L.J.; Li, Q.; Sun, L.; Chiarelli, A.M.; Hislop, G.; Yaffe, M.J.; Minkin, S. Mammographic density as a surrogate marker for the effects of hormone therapy on risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2006, 15, 961–966. [Google Scholar]
- Boyd, N.F.; Martin, L.J.; Sun, L.; Guo, H.; Chiarelli, A.; Hislop, G.; Yaffe, M.; Minkin, S. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 2086–2092. [Google Scholar]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar]
- Petrakis, N.L.; Barnes, S.; King, E.B.; Lowenstein, J.; Wiencke, J.; Lee, M.M.; Miike, R.; Kirk, M.; Coward, L. Stimulatory influence of soy protein isolate on breast secretion in pre-and postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 785–794. [Google Scholar]
- Hargreaves, D.F.; Potten, C.S.; Harding, C.; Shaw, L.E.; Morton, M.S.; Roberts, S.A.; Howell, A.; Bundred, N.J. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J. Clin. Endocrinol. Metab 1999, 84, 4017–4024. [Google Scholar]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar]
- Boyd, N.; Martin, L.; Chavez, S.; Gunasekara, A.; Salleh, A.; Melnichouk, O.; Yaffe, M.; Friedenreich, C.; Minkin, S.; Bronskill, M. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009, 10, 569–580. [Google Scholar]
- Glide, C.; Duric, N.; Littrup, P. Novel approach to evaluating breast density utilizing ultrasound tomography. Med. Phys. 2007, 34, 744–753. [Google Scholar]
- Shepherd, J.A.; Malkov, S.; Fan, B.; Laidevant, A.; Novotny, R.; Maskarinec, G. Breast density assessment in adolescent girls using dual-energy X-ray absorptiometry: a feasibility study. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1709–1713. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maskarinec, G.; Verheus, M.; A. Tice, J. Epidemiologic Studies of Isoflavones & Mammographic Density. Nutrients 2010, 2, 35-48. https://doi.org/10.3390/nu2010035
Maskarinec G, Verheus M, A. Tice J. Epidemiologic Studies of Isoflavones & Mammographic Density. Nutrients. 2010; 2(1):35-48. https://doi.org/10.3390/nu2010035
Chicago/Turabian StyleMaskarinec, Gertraud, Martijn Verheus, and Jeffrey A. Tice. 2010. "Epidemiologic Studies of Isoflavones & Mammographic Density" Nutrients 2, no. 1: 35-48. https://doi.org/10.3390/nu2010035