Antioxidant Properties and Neuroprotective Capacity of Strawberry Tree Fruit (Arbutus unedo)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenols, Anthocyaninsand Peroxyl Scavenging Activity
| Fruit | Total phenol content (mg GAE g-1 dw) | Anthocyanin content (mg cy-3-glucoside. 100 g-1 dw) | Antioxidant activity (mmol TE. 100 g-1 dw) |
|---|---|---|---|
| A. unedo | 16.46 ± 3.66 | 76.26 ± 9.85*** | 11.66 ± 2.01 |
| R. idaeus | 13.23 ± 0.94 | 438.60 ± 12.20 | 15.37 ± 2.73 |
2.2. Effect of Fruit Extracts on a Neurodegeneration Cell Model
 A. unedo
A. unedo  R. idaeus. Each point is the average of three independent replicates. Vertical bars represent ± SD.
R. idaeus. Each point is the average of three independent replicates. Vertical bars represent ± SD.
 A. unedo
A. unedo  R. idaeus. Each point is the average of three independent replicates. Vertical bars represent ± SD.
R. idaeus. Each point is the average of three independent replicates. Vertical bars represent ± SD. 
 ) 50, (
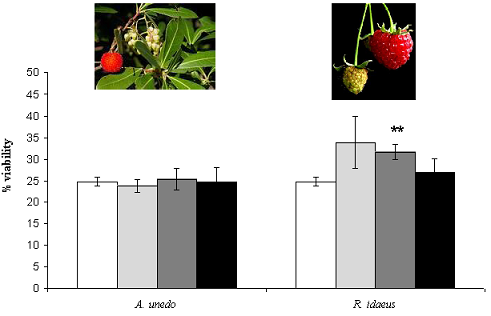
) 50, (  ) 125 and (■) 175 µg GAE .mL-1. Each point is the average of three independent replicates. Vertical bars represent ± SD. ** - significantly different values for p < 0.01 .
) 125 and (■) 175 µg GAE .mL-1. Each point is the average of three independent replicates. Vertical bars represent ± SD. ** - significantly different values for p < 0.01 .
 ) 50, (
) 50, (  ) 125 and (■) 175 µg GAE .mL-1. Each point is the average of three independent replicates. Vertical bars represent ± SD. ** - significantly different values for p < 0.01 .
) 125 and (■) 175 µg GAE .mL-1. Each point is the average of three independent replicates. Vertical bars represent ± SD. ** - significantly different values for p < 0.01 .
3. Experimental Section
3.1. Biological Material
3.2. Extract Preparation
3.3. Total Phenolic Measurement
3.4. Anthocyanin Content
3.5. Peroxyl Radical Scavenging Capacity Assay
3.6. HPLC-MS Phenolic Profile Determination
3.7. Cell Culture
3.8. Cell Viability
3.9. Intracellular Antioxidant Activity
3.10. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Dore, S. Unique properties of polyphenol stilbenes in the brain: More than direct antioxidant actions; gene/protein regulatory activity. Neurosignals 2005, 14, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar]
- Butterfield, D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in alzheimer's disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bickford, P.C.; Gould, T.; Briederick, L.; Chadman, K.; Pollock, A.; Young, D., et al. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000, 866, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. Beneficial effects of berry fruit polyphenols on neuronal and behavioral aging. J. Sci. Food Agric. 2006, 86, 2251–2255. [Google Scholar]
- Shukitt-Hale, B.; Lau, F.C.; Joseph, J.A. Berry fruit supplementation and the aging brain. J. Agric. Food Chem. 2008, 56, 636–641. [Google Scholar]
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, non-volatile and phenolic acids composition of strawberry tree (Arbutus unedo L. var.Ellipsoidea ) fruits. J. Food Comp. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Alarcao-E-Silva, M.L.C.M.M.; Leitao, A.E.B.; Azinheira, H.G.; Leitao, M.C.A. The Arbutus berry: Studies on its color and chemical characteristics at two mature stages. J. Food Comp. Anal. 2001, 14, 27–35. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Comp. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Bnouham, M.; Merhfour, F.Z.; Legssyer, A.; Mekhfi, H.; Maallem, S.; Ziyyat, A. Antihyperglycemic activity of Arbutus unedo, Ammoides pusilla and Thymelaea hirsuta. Pharmazie. 2007, 62, 630–632. [Google Scholar] [PubMed]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus unedo L. (ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef] [PubMed]
- Males, Z.; Plazibat, M.; Vundac, V.B.; Zuntar, I. Qualitative and quantitative analysis of flavonoids of the strawberry tree-Arbutus unedo L. (ericaceae). Acta Pharm. 2006, 56, 245–250. [Google Scholar] [PubMed]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar]
- Rouanet, J.-M.; Décordé, K.; Rio, D.D.; Auger, C.; Borges, G.; Cristol, J.-P.; Lean, M.E.J.; Crozier, A. Berry juices, teas, antioxidants and the prevention of atherosclerosis in hamsters. Food Chem. 2010, 118, 266–271. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem. in Press.
- Linden, A.; Gulden, M.; Martin, H.J.; Maser, E.; Seibert, H. Peroxide-induced cell death and lipid peroxidation in c6 glioma cells. Toxicol. In Vitro 2008, 22, 1371–1376. [Google Scholar]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- García-Alonso, M.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; van der Meer, I.M.; Ric de Vos, C.H. Antioxidants in raspberry: On-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar]
- Trewavas, A.; Stewart, D. Paradoxical effects of chemicals in the diet on health. Curr. Opin. Plant Biol. 2003, 6, 185–190. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001, 22, 285–291. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health. 2001, 22, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Applications of hormesis in toxicology, risk assessment and chemotherapeutics. Trends Pharmacol. Sci. 2002, 23, 331–337. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Rad. Biol. Med. 1993, 14, 303–311. [Google Scholar]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G., et al. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
Appendix
| Peak No. | RT | PDA | M/Z [M-H] | MS2 | Putative identity |
|---|---|---|---|---|---|
| 1 | 7.37 | 280 | 331.1 | 271.0, 211.1, 169.0 | Gallic acid glucoside |
| 2 | 8.7 | 265 | 331.1 | 169.0, 125.0 | Galloyl glucoside |
| 3 | 10.64 | 270 | 343 | 191.2, 169.0 | 3-O- or 5-O-galloylquinic acid [12] |
| 4 | 14.04 | 255-300 | 331.1 | 169.1 | Gallic acid 4-O-B-D-glucopyranoside or B-D-Glucogalline [12] |
| 5 | 16.29 | 255-300 | 325 | 169.0, 125.1 | Galloyl shikimic acid |
| 6 | 20.93 | 280 | 577.1 | 289.2 | Proanthocyanidin dimer [12] |
| 7 | 21.36 | 270-290 | 495.0, 465.0, 343.0, 191.2 | 343.0, 191.0 | Digalloylquinic acid |
| 8 | 21.95 | 295 | 495.0, 465.0, 343.0, 191.2 | 343.0, 191.0 | Isomer of digalloylquinic acid |
| 9 | 22.66 | 280 | 577.0, 423.2, 407.2, 289.2 | 425.0, 407.2, 289.2 | Procyanidin dimer B2 [10] |
| 10 | 23.86 | 280 | 289.1 | 261.0, 175.0 | Catechin |
| 11 | 24.31 | 320 | 865.1, 453.2, 325.1 | 577.1 | Procyanidin trimer |
| 12 | 24.6 | 285 | 541.1, 483.1, 467.3, 321.0, 301.2 | 453.1, 301.4, 169.2 | Gallic acid derivative |
| 13 | 25.5 | 280, 525 | 477.0, 325.1 | 325.0, 169.0 | Digalloyl shikimic acid |
| 14 | 26.1 | 275 | 477.0, 325.1 | 325.0, 169.0 | Digalloyl shikimic acid |
| 15 | 26.84 | 270 | 633.1, 463.1, 301.2, 275.2 | 463.0, 301.1 | Strictinin ellagitannin |
| 16 | 28.68 | 360 | 463.2, 301.3 | 301.2 | Quercetin-3-glucoside |
| 17 | 29.44 | 275 | 1109.0, 972.9, 647.0, 635.1, 588.1, 441.0, 301.3 | 783.1, 492.8 | Gallotannin derivative |
| 18 | 31.09 | 280 | 366.2, 186.0 | 204.1, 186.1, 142.0 | Unknown |
| 19 | 33.08 | 275 | 953 | 633.0, 463.2, 301.2 | Tannin |
| 20 | 33.36 | 360 | 433.1, 301.2 | 301 | Quercetin-3-xyloside |
| 21 | 34.67 | 260-355 | 615.2, 463.2, 433.1, 301.1 | 463.0, 301.1 | Quercetin hexose galloyl derivative |
| 22 | 35.21 | 260-355 | 615.2, 463.2, 433.1, 301.1 | 463.0, 301.1 | Quercetin hexose galloyl derivative |
| 23 | 36.3 | 260-355 | 615.2, 463.2, 433.1, 301.1 | 463.0, 301.1 | Quercetin hexose galloyl derivative |
| 24 | 36.69 | 255, 370 | 301.2 | 301.2, 257.2 | Ellagic acid |
| 25 | 36.96 | 345 | 463.1, 301.2 | 301.2 | Quercetin 3-galactoside |
| 26 | 37.4 | 275-355 | 463.1, 301.2 | 301.2 | Quercetin 3-glucoside |
| 27 | 38.09 | 280 | 939.1, 769.1, 729.0, 617.1, 544.2, 480.2, 469.2 | 769.0, 617.2 | Gallotannin |
| 28 | 39.81 | 355 | 599.0, 301.0 | 463.1, 301.1 | Ellagic acid-hexose derivative |
| 29 | 40.75 | 355 | 433.0, 301.1 | 301.1 | Ellagic acid arabinoside/xyloside |
| 30 | 41.49 | 355 | 447.0, 301.1 | 301.1 | Ellagic acid rhamnoside |
| Peak No. | RT | PDA | M/Z [M+H] | MS2 | Putative identity |
|---|---|---|---|---|---|
| A1 | 20.60 | 280, 525 | 579.1, 465.1, 303.2 | 303.2 | Delphinidin-3-glucoside or delphinidin-3-galactoside [10] |
| A2 | 22.94 | 280, 515 | 449.0, 287.2 | 287.2 | Cyanidin 3-O-glucoside or cyanidin-3-galactoside [10] |
| A3 | 25.28 | 280, 515 | 419.1, 287.2 | 287.2 | Cyanidin 3-O-arabinoside [10] |

| Peak No. | RT | PDA | M/Z [M-H] | MS2 | Putative identity |
|---|---|---|---|---|---|
| 1 | 23.03 | 265 | 1250.8, 609.3, 444.9, 323.0, 301.2, 224.83, 136.7 | ND | Ellagitannin |
| 2 | 24.43 | 340 | 627.1, 593.1, 491.2, 285.1 | 517.1, 285.1 | Unknown |
| 3 | 25.68 | 280, 365 | 577.1, 465.1, 407.2, 289.2 | 289.2 | Proanthocyanidin dimer |
| 4 | 27.25 | 260 | 1566.9, 1265.0, 783.3, 633.2, 301.2 | 481.0, 301.2 | Ellagitannin |
| 5 | 28.04 | 280, 340 | 289.2 | 289.0, 245.1 | Epicatechin |
| 6 | 30.36 | 250 | 1868.9, 1567.0, 1401.3, 1250.3, 934.1, 633.1, 301.2 | 1567.0, 1250.0, 932.6, 897.0 | Lambertianin C |
| 7 | 31.16 | 360 | 625.2, 301.2 | 301.1 | Ellagic acid diglucoside |
| 8 | 31.50 | 250 | 1868.9, 1567.0, 1235.0, 934.3, 633.2, 301.2 | 1566.9, 1235.2, 933.1, 897.0 | Sanguiin H6 |
| 9 | 33.22 | 360 | 433.1, 301.2 | 301.2 | Ellagic acid arabinoside |
| 10 | 36.57 | 370 | 301.2 | 301.2 | Ellagic acid |
| 11 | 37.31 | 345 | 477.0, 463.1, 301.2 | 301.2 | Quercetin-3-glucuronide |
| 12 | 39.62 | 360 | 447.1, 315.1, 300.1 | 315.1 | Methyl ellagic acid conjugate |
| Peak No. | RT | PDA | M/Z [M+H] | MS2 | Putative identity |
|---|---|---|---|---|---|
| A1 | 26.17 | 280,515 | 611.1 | 287.2 | Cyanidin-3-sophoroside |
| A2 | 27.79 | 275, 515 | 595.1 | 449.1, 287.3 | Cyanidin-3-rutinoside |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fortalezas, S.; Tavares, L.; Pimpão, R.; Tyagi, M.; Pontes, V.; Alves, P.M.; McDougall, G.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant Properties and Neuroprotective Capacity of Strawberry Tree Fruit (Arbutus unedo). Nutrients 2010, 2, 214-229. https://doi.org/10.3390/nu2020214
Fortalezas S, Tavares L, Pimpão R, Tyagi M, Pontes V, Alves PM, McDougall G, Stewart D, Ferreira RB, Santos CN. Antioxidant Properties and Neuroprotective Capacity of Strawberry Tree Fruit (Arbutus unedo). Nutrients. 2010; 2(2):214-229. https://doi.org/10.3390/nu2020214
Chicago/Turabian StyleFortalezas, Sofia, Lucélia Tavares, Rui Pimpão, Meenu Tyagi, Vera Pontes, Paula M. Alves, Gordon McDougall, Derek Stewart, Ricardo B. Ferreira, and Cláudia N. Santos. 2010. "Antioxidant Properties and Neuroprotective Capacity of Strawberry Tree Fruit (Arbutus unedo)" Nutrients 2, no. 2: 214-229. https://doi.org/10.3390/nu2020214