Vitamin D Interactions with Soy Isoflavones on Bone after Menopause: A Review
Abstract
:1. Introduction
2. Vitamin D and Ca Absorption
3. Vitamin D and BMD
4. Soy Isoflavones and Bone
5. Soy Isoflavones, Vitamin D and Bone
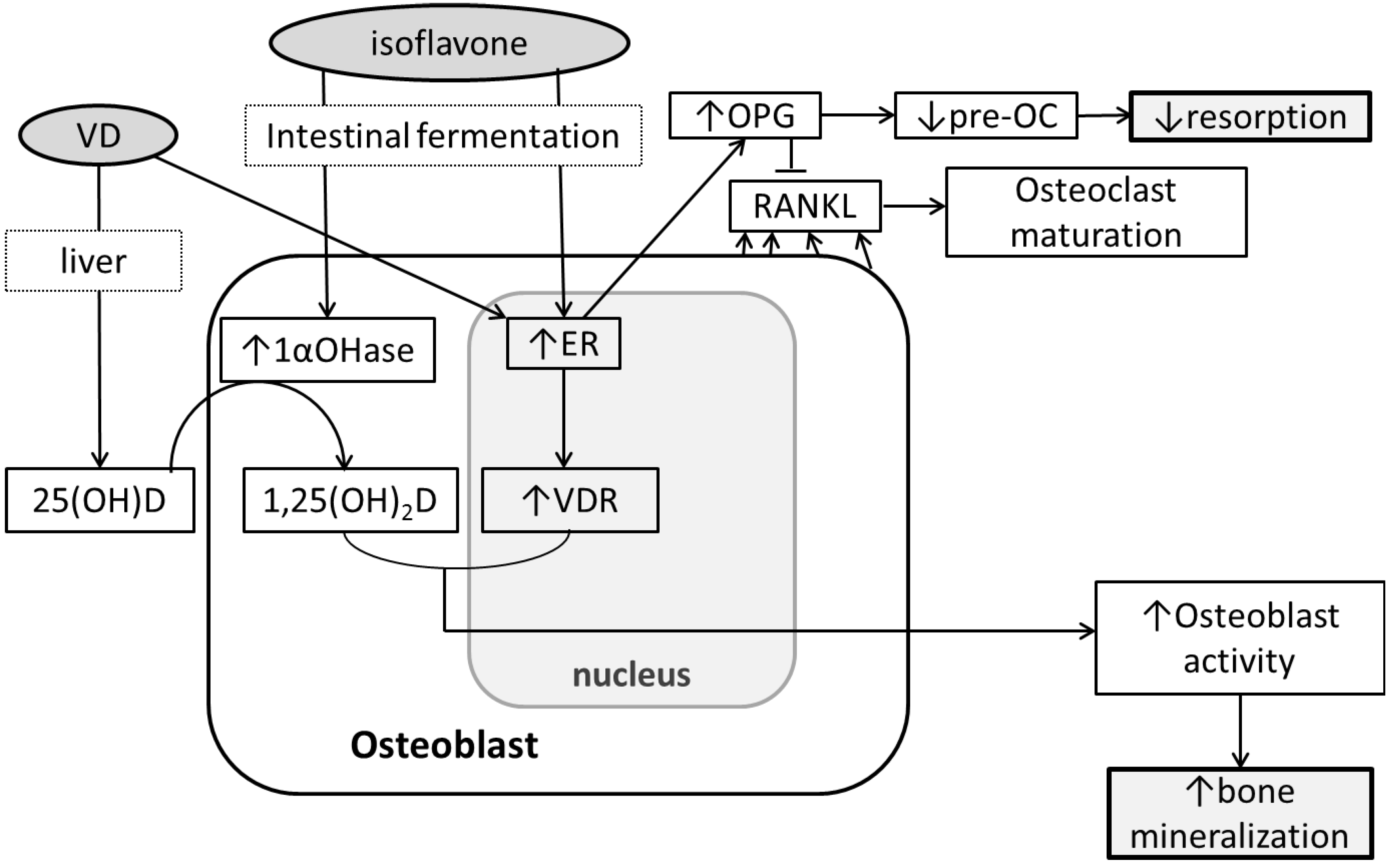
6. Conclusions
Conflict of Interest
References
- Pubmed Health: Osteoporosis—Overview. Available online: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001400/ (accessed on 22 March 2012).
- What Is Osteoporosis? Available online: http://www.nof.org/articles/7 (accessed on 15 November 2012).
- Russo, A.; Holmquist, L.; Elixhauser, A. U.S. Hospitalizations Involving Osteoporosis and Injury, 2006; Statistical Brief #76; Agency for Health Care Policy and Research (US): Rockville, MD, USA, 2009. [Google Scholar]
- Heaney, R.P.; Weaver, C.M. Calcium and vitamin D. Endocrinol. Metab. Clin. North Am. 2003, 32, 181–194. [Google Scholar] [CrossRef]
- Schuit, S.C.; van der Klift, M.; Weel, A.E.; de Laet, C.E.; Burger, H.; Seeman, E.; Hofman, A.; Uitterlinden, A.G.; van Leeuwen, J.P.; Pols, H.A. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004, 34, 195–202. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Heaney, R.P.; Barger-Lux, M.J.; Dowell, M.S.; Chen, T.C.; Holick, M.F. Calcium absorptive effects of vitamin D and its major metabolites. J. Clin. Endocrinol. Metab. 1997, 2, 4111–4116. [Google Scholar]
- Francis, R.M.; Peacock, M. Local action of oral 1,25-dihydroxycholecalciferol on calcium absorption in osteoporosis. Am. J. Clin. Nutr. 1987, 46, 315–318. [Google Scholar]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar]
- Need, A.G.; Nordin, B.E. Misconceptions—vitamin D insufficiency causes malabsorption of calcium. Bone 2008, 42, 1021–1024. [Google Scholar] [CrossRef]
- Need, A.G.; O’Loughlin, P.D.; Morris, H.A.; Coates, P.S.; Horowitz, M.; Nordin, B.E. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J. Bone Miner. Res. 2008, 23, 1859–1863. [Google Scholar] [CrossRef]
- Hansen, K.E.; Jones, A.N.; Lindstrom, M.J.; Davis, L.A.; Engelke, J.A.; Shafer, M.M. Vitamin D insufficiency: Disease or no disease? J. Bone Miner. Res. 2008, 23, 1052–1060. [Google Scholar] [CrossRef]
- Zhu, K.; Devine, A.; Dick, I.M.; Wilson, S.G.; Prince, R.L. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: A five-year randomized controlled trial. J. Clin. Endocrinol. Metab. 2008, 93, 743–749. [Google Scholar]
- Aloia, J.F.; Chen, D.G.; Yeh, J.K.; Chen, H. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am. J. Clin. Nutr. 2010, 92, 835–840. [Google Scholar] [CrossRef]
- Need, A.G.; Kemp, A.; Giles, N.; Morris, H.A.; Horowitz, M.; Nordin, B.E. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos. Int. 2002, 13, 83–88. [Google Scholar] [CrossRef]
- Schnatz, P.F.; Marakovits, K.A.; O’Sullivan, D.M.; Ethun, K.; Clarkson, T.B.; Appt, S.E. Response to an adequate dietary intake of vitamin D3 modulates the effect of estrogen therapy on bone density. J. Womens Health (Larchmt.) 2012, 21, 858–864. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Kiel, D.P.; Dawson-Hughes, B.; Orav, J.E.; Li, R.; Spiegelman, D.; Dietrich, T.; Willett, W.C. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J. Bone Miner. Res. 2009, 24, 935–942. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011.
- Viljakainen, H.T.; Natri, A.M.; Kärkkäinen, M.; Huttunen, M.M.; Palssa, A.; Jakobsen, J.; Cashman, K.D.; Mølgaard, C.; Lamberg-Allardt, C. A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: A double-blinded randomized placebo-controlled 1-year intervention. J. Bone Miner. Res. 2006, 21, 836–844. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hicks, P.D.; Hawthorne, K.M. Higher serum 25-hydroxyvitamin D levels in school-age children are inconsistently associated with increased calcium absorption. J. Clin. Endocrinol. Metab. 2009, 94, 2421–2427. [Google Scholar] [CrossRef]
- Lee, W.T.; Cheng, J.C.; Jiang, J.; Hu, P.; Hu, X.; Roberts, D.C. Calcium absorption measured by stable calcium isotopes (42Ca & 44Ca) among Northern Chinese adolescents with low vitamin D status. J. Orthop. Surg. (Hong Kong) 2002, 10, 61–66. [Google Scholar]
- Park, C.Y.; Hill, K.M.; Elble, A.E.; Martin, B.R.; DiMeglio, L.A.; Peacock, M.; McCabe, G.P.; Weaver, C.M. Daily supplementation with 25 μg cholecalciferol does not increase calcium absorption or skeletal retention in adolescent girls with low serum 25-hydroxyvitamin D. J. Nutr. 2010, 140, 2139–2144. [Google Scholar] [CrossRef]
- Howard, G.A.; Turner, R.T.; Sherrard, D.J.; Baylink, D.J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J. Biol. Chem. 1981, 256, 7738–7740. [Google Scholar]
- Van Leeuwen, J.P.; van Driel, M.; van den Bemd, G.J.; Pols, H.A. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit. Rev. Eukaryot. Gene Expr. 2001, 11, 199–226. [Google Scholar]
- Gardiner, E.M.; Baldock, P.A.; Thomas, G.P.; Sims, N.A.; Henderson, N.K.; Hollis, B.; White, C.P.; Sunn, K.L.; Morrison, N.A.; Walsh, W.R.; Eisman, J.A. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000, 14, 1908–1916. [Google Scholar] [CrossRef]
- Anderson, P.H.; Sawyer, R.K.; Moore, A.J.; May, B.K.; O’Loughlin, P.D.; Morris, H.A. Vitamin D depletion induces RANKL-mediated osteoclastogenesis and bone loss in a rodent model. J. Bone Miner. Res. 2008, 23, 1789–1797. [Google Scholar] [CrossRef]
- Fleet, J.C.; Gliniak, C.; Zhang, Z.; Xue, Y.; Smith, K.B.; McCreedy, R.; Adedokun, S.A. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J. Nutr. 2008, 138, 1114–1120. [Google Scholar]
- Hohman, E.E.; Martin, B.R.; Lachcik, P.J.; Gordon, D.T.; Fleet, J.C.; Weaver, C.M. Bioavailability and efficacy of vitamin D2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J. Agric. Food Chem. 2011, 59, 2341–2346. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Ichimura, S.; Sato, Y.; Yeh, J.K. Differential effect of vitamin K and vitamin D supplementation on bone mass in young rats fed normal or low calcium diet. Yonsei Med. J. 2004, 45, 314–324. [Google Scholar]
- Peacock, M.; Liu, G.; Carey, M.; McClintock, R.; Ambrosius, W.; Hui, S.; Johnston, C.C. Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J. Clin. Endocrinol. Metab. 2000, 85, 3011–3019. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Dallal, G.E.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Grant, A.M.; Avenell, A.; Campbell, M.K.; McDonald, A.M.; MacLennan, G.S.; McPherson, G.C.; Anderson, F.H.; Cooper, C.; Francis, R.M.; Donaldson, C.; et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): A randomised placebo-controlled trial. Lancet 2005, 365, 1621–1628. [Google Scholar]
- Chung, M.; Balk, E.M.; Brendel, M.; Ip, S.; Lau, J.; Lee, J.; Lichtenstein, A.; Patel, K.; Raman, G.; Tatsioni, A.; et al. Vitamin D and Calcium: A Systematic Review of Health Outcomes; Evidence Reports/Technology Assessments, No. 183, Agency for Healthcare Research and Quality: Rockville, MD, USA, August 2009.
- Venken, K.; Callewaert, F.; Boonen, S.; Vanderschueren, D. Sex hormones, their receptors and bone health. Osteoporos. Int. 2008, 19, 1517–1525. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef]
- Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, 2004.
- Riggs, B.L.; Khosla, S.; Atkinson, E.J.; Dunstan, C.R.; Melton, L.J. Evidence that type I osteoporosis results from enhanced responsiveness of bone to estrogen deficiency. Osteoporos. Int. 2003, 14, 728–733. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. Am. J. Med. 1998, 104, 219–226. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Li, Y.Q.; Xing, X.H.; Wang, H.; Weng, X.L.; Yu, S.B.; Dong, G.Y. Dose-dependent effects of genistein on bone homeostasis in rats’ mandibular subchondral bone. Acta Pharmacol. Sin. 2012, 33, 66–74. [Google Scholar] [CrossRef]
- Cotter, A.A.; Cashman, K.D. Lack of dose-responsive effect of dietary phyto-oestrogens on transepithelial calcium transport in human intestinal-like Caco-2 cells. Br. J. Nutr. 2004, 91, 5–9. [Google Scholar] [CrossRef]
- Cotter, A.A.; Jewell, C.; Cashman, K.D. The effect of oestrogen and dietary phyto-oestrogens on transepithelial calcium transport in human intestinal-like Caco-2 cells. Br. J. Nutr. 2003, 89, 755–765. [Google Scholar] [CrossRef]
- Cheong, J.M.; Martin, B.R.; Jackson, G.S.; Elmore, D.; McCabe, G.P.; Nolan, J.R.; Barnes, S.; Peacock, M.; Weaver, C.M. Soy isoflavones do not affect bone resorption in postmenopausal women: A dose-response study using a novel approach with 41Ca. J. Clin. Endocrinol. Metab. 2007, 92, 577–582. [Google Scholar]
- Weaver, C.M.; Martin, B.R.; Jackson, G.S.; McCabe, G.P.; Nolan, J.R.; McCabe, L.D.; Barnes, S.; Reinwald, S.; Boris, M.E.; Peacock, M. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using 41Ca methodology. J. Clin. Endocrinol. Metab. 2009, 94, 3798–3805. [Google Scholar]
- Mun, J.G.; Grannan, M.D.; Lachcik, P.J.; Rogers, R.B.; Yousef, G.G.; Grace, M.H.; Janle, E.M.; Wu, Q.L.; Simon, J.E.; Weaver, C.M.; Lila, M.A. Tracking deposition of a 14C-radiolabeled kudzu hairy root-derived isoflavone-rich fraction into bone. Exp. Biol. Med. (Maywood) 2010, 235, 1224–1235. [Google Scholar] [CrossRef]
- De Wilde, A.; Lieberherr, M.; Colin, C.; Pointillart, A. A low dose of daidzein acts as an ERbeta-selective agonist in trabecular osteoblasts of young female piglets. J. Cell. Physiol. 2004, 200, 253–262. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.-S.; Lothy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Rassi, C.M.; Lieberherr, M.; Chaumaz, G.; Pointillart, A.; Cournot, G. Down-regulation of osteoclast differentiation by daidzein via caspase 3. J. Bone Miner. Res. 2002, 17, 630–638. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J. Bone Miner. Res. 2008, 23, 715–720. [Google Scholar] [CrossRef]
- Legette, L.L.; Lee, W.H.; Martin, B.R.; Story, J.A.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Genistein, a phytoestrogen, improves total cholesterol, and Synergy, a prebiotic, improves calcium utilization, but there were no synergistic effects. Menopause 2011, 18, 923–931. [Google Scholar] [CrossRef]
- Mathey, J.; Mardon, J.; Fokialakis, N.; Puel, C.; Kati-Coulibaly, S.; Mitakou, S.; Bennetau-Pelissero, C.; Lamothe, V.; Davicco, M.J.; Lebecque, P.; et al. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: The case for equol. Osteoporos. Int. 2007, 18, 671–679. [Google Scholar]
- Phrakonkham, P.; Chevalier, J.; Desmetz, C.; Pinnert, M.F.; Bergès, R.; Jover, E.; Davicco, M.J.; Bennetau-Pelissero, C.; Coxam, V.; Artur, Y.; Canivenc-Lavier, M.C. Isoflavonoid-based bone-sparing treatments exert a low activity on reproductive organs and on hepatic metabolism of estradiol in ovariectomized rats. Toxicol. Appl. Pharmacol. 2007, 224, 105–115. [Google Scholar] [CrossRef]
- Legette, L.L.; Martin, B.R.; Shahnazari, M.; Lee, W.H.; Helferich, W.G.; Qian, J.; Waters, D.J.; Arabshahi, A.; Barnes, S.; Welch, J.; et al. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J. Nutr. 2009, 139, 1908–1913. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar]
- Potter, S.M.; Baum, J.A.; Teng, H.; Stillman, R.J.; Shay, N.F.; Erdman, J.W. Soy protein and isoflavones: Their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998, 68, 1375–1379. [Google Scholar]
- Wong, W.W.; Lewis, R.D.; Steinberg, F.M.; Murray, M.J.; Cramer, M.A.; Amato, P.; Young, R.L.; Barnes, S.; Ellis, K.J.; Shypailo, R.J.; et al. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am. J. Clin. Nutr. 2009, 90, 1433–1439. [Google Scholar] [CrossRef]
- Tai, T.Y.; Tsai, K.S.; Tu, S.T.; Wu, J.S.; Chang, C.I.; Chen, C.L.; Shaw, N.S.; Peng, H.Y.; Wang, S.Y.; Wu, C.H. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporos. Int. 2012, 23, 1571–1580. [Google Scholar] [CrossRef]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar]
- Franke, A.A.; Lai, J.F.; Halm, B.M.; Pagano, I.; Kono, N.; Mack, W.J.; Hodis, H.N. Equol production changes over time in postmenopausal women. J. Nutr. Biochem. 2012, 23, 573–579. [Google Scholar] [CrossRef]
- Gilad, L.A.; Tirosh, O.; Schwartz, B. Phytoestrogens regulate transcription and translation of vitamin D receptor in colon cancer cells. J. Endocrinol. 2006, 191, 387–398. [Google Scholar] [CrossRef]
- Liel, Y.; Kraus, S.; Levy, J.; Shany, S. Evidence that estrogens modulate activity and increase the number of 1,25-dihydroxyvitamin D receptors in osteoblast-like cells (ROS 17/2.8). Endocrinology 1992, 130, 2597–2601. [Google Scholar] [CrossRef]
- Ishibe, M.; Nojima, T.; Ishibashi, T.; Koda, T.; Kaneda, K.; Rosier, R.N.; Puzas, J.E. 17 beta-estradiol increases the receptor number and modulates the action of 1,25-dihydroxyvitamin D3 in human osteosarcoma-derived osteoblast-like cells. Calcif. Tissue Int. 1995, 57, 430–435. [Google Scholar] [CrossRef]
- Somjen, D.; Katzburg, S.; Kohen, F.; Gayer, B.; Sharon, O.; Hendel, D.; Posner, G.H.; Kaye, A.M. Responsiveness to phytoestrogens in primary human osteoblasts is modulated differentially by a “less-calcemic” analog of 1,25 dihydroxyvitamin D3: JK 1624F2-2 (JKF). J. Steroid Biochem. Mol. Biol. 2006, 98, 139–146. [Google Scholar] [CrossRef]
- Chennaiah, S.; Vijayalakshmi, V.; Suresh, C. Effect of the supplementation of dietary rich phytoestrogens in altering the vitamin D levels in diet induced osteoporotic rat model. J. Steroid Biochem. Mol. Biol. 2010, 121, 268–272. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Zhou, Y. A synergetic role of 1,25-dihydroxyvitamin D3 in 17β-estradial induced-proliferation and differentiation of osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2011, 659, 273–280. [Google Scholar] [CrossRef]
- Tuppurainen, M.T.; Komulainen, M.; Kröger, H.; Honkanen, R.; Jurvelin, J.; Puntila, E.; Heikkinen, A.M.; Alhava, E.; Saarikoski, S. Does vitamin D strengthen the increase in femoral neck BMD in osteoporotic women treated with estrogen? Osteoporos. Int. 1998, 8, 32–38. [Google Scholar] [CrossRef]
- Bitto, A.; Marini, H.; Burnett, B.P.; Polito, F.; Levy, R.M.; Irrera, N.; Minutoli, L.; Adamo, E.B.; Squadrito, F.; Altavilla, D. Genistein aglycone effect on bone loss is not enhanced by supplemental calcium and vitamin D3: A dose ranging experimental study. Phytomedicine 2011, 18, 879–886. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Park, C.Y.; Weaver, C.M. Vitamin D Interactions with Soy Isoflavones on Bone after Menopause: A Review. Nutrients 2012, 4, 1610-1621. https://doi.org/10.3390/nu4111610
Park CY, Weaver CM. Vitamin D Interactions with Soy Isoflavones on Bone after Menopause: A Review. Nutrients. 2012; 4(11):1610-1621. https://doi.org/10.3390/nu4111610
Chicago/Turabian StylePark, Clara Y., and Connie M. Weaver. 2012. "Vitamin D Interactions with Soy Isoflavones on Bone after Menopause: A Review" Nutrients 4, no. 11: 1610-1621. https://doi.org/10.3390/nu4111610



