Red-Koji Fermented Red Ginseng Ameliorates High Fat Diet-Induced Metabolic Disorders in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation and Administration of Test Materials
2.3. Measurement of Body and Organ Weights
2.4. Measurement of Mean Daily Food Consumption
2.5. Blood Biochemistry
2.6. Histopathology
2.7. Statistical Analyses
3. Results
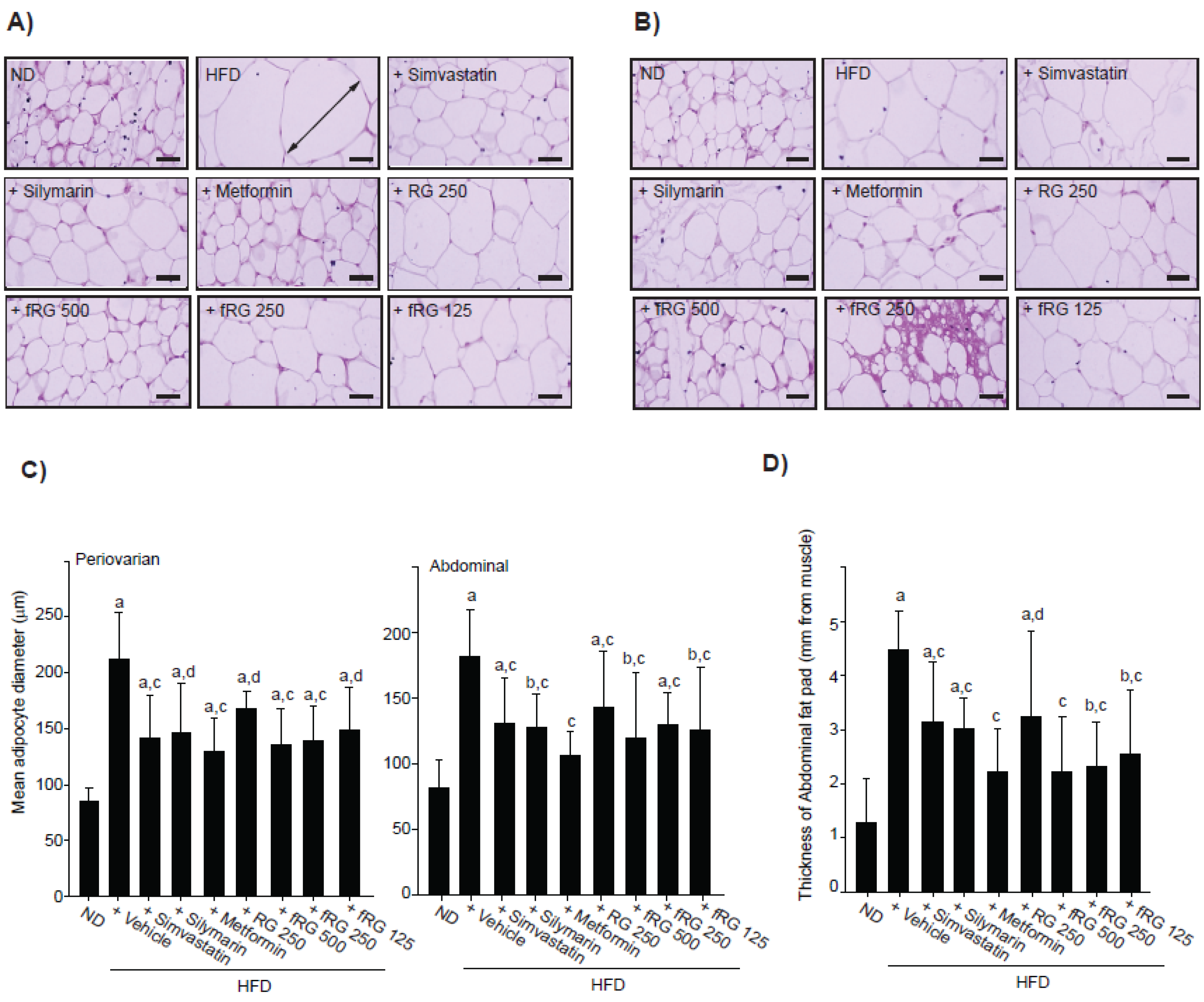
3.1. Effect of fRG on HFD-Induced Obesity
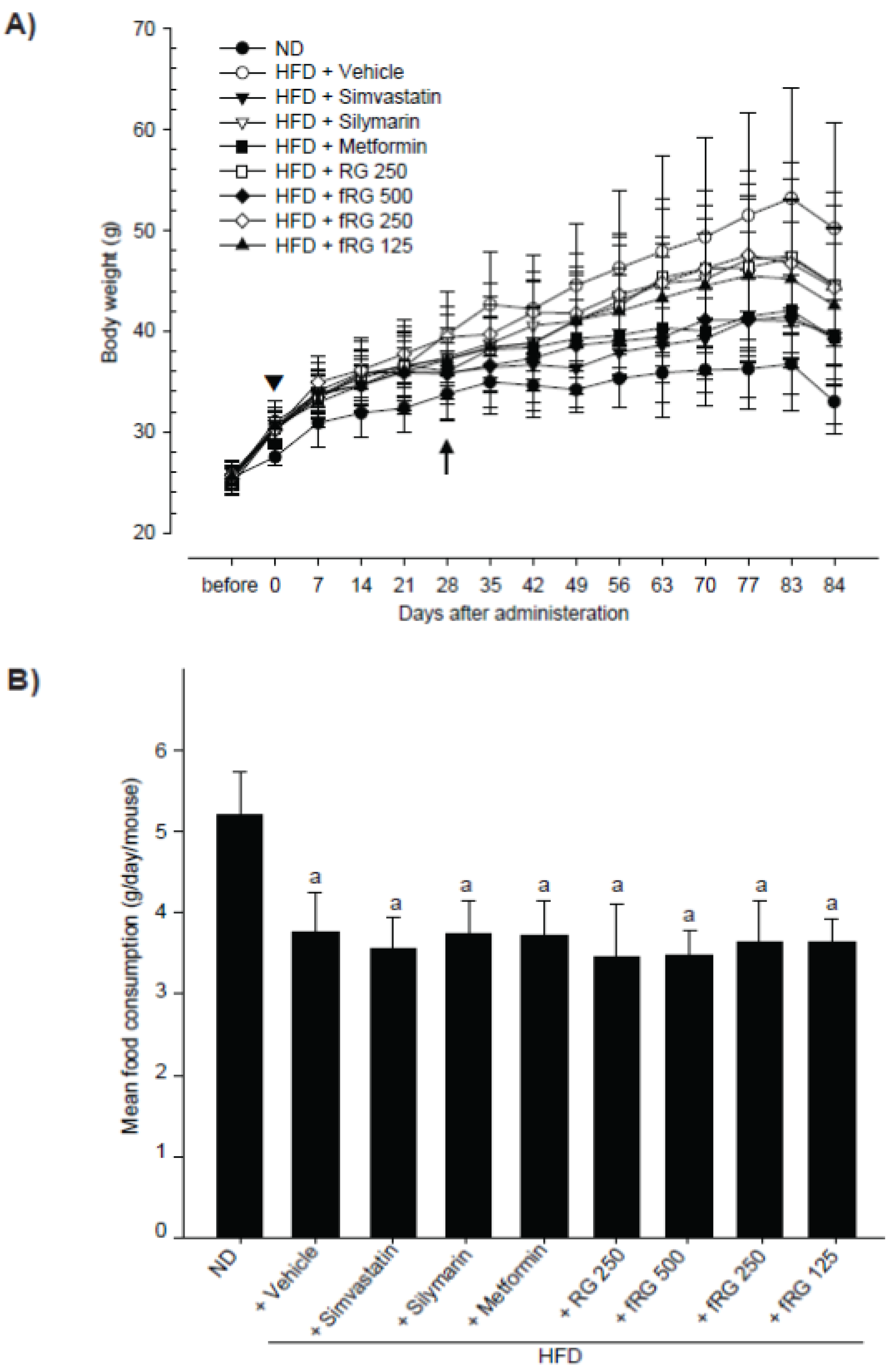
| Groups | Body weights (g): | Body weight gains during (g): | ||
|---|---|---|---|---|
| At start of administration | At a termination | Adapt period (7 days) | Administration period (84 days) | |
| ND | 27.54 ± 0.88 | 33.01 ± 2.28 | 2.04 ± 0.63 | 5.48 ± 1.58 |
| HFD | 31.04 ± 2.12 a | 50.20 ± 10.44 a | 5.63 ± 1.36 a | 19.16 ± 11.02 a |
| +Simvastatin | 30.40 ± 1.90 a | 39.70 ± 3.28 a,c | 4.51 ± 0.93 a | 9.33 ± 2.82 b,c |
| +Silymarin | 30.24 ± 0.94 a | 44.61 ± 7.88 a | 5.38 ± 1.14 a | 14.38 ± 7.53 a |
| +Metformin | 30.41 ± 1.71 a | 39.31 ± 4.81 ac | 5.09 ± 1.25 a | 8.90 ± 4.36 c |
| +RG 250 mg/kg | 30.29 ± 1.22 a | 44.54 ± 9.23 a | 4.54 ± 0.76 a | 14.25 ± 8.33 b |
| +fRG 500 mg/kg | 30.19 ± 1.19 a | 39.28 ± 9.48 | 4.84 ± 0.86 a | 9.09 ± 9.27 |
| +fRG 250 mg/kg | 30.30 ± 1.11 a | 44.31 ± 5.81 a | 4.58 ± 1.16 a | 14.01 ± 5.66 a |
| +fRG 125 mg/kg | 30.58 ± 1.29 a | 42.56 ± 7.83 a | 4.94 ± 1.00 a | 11.99 ± 8.86 |
| Groups | Periovarian fat | Liver | Kidney | |||
|---|---|---|---|---|---|---|
| Absolute (g) | Relative (% of body weights) | Absolute (g) | Relative (% of body weights) | Absolute (g) | Relative (% of body weights) | |
| ND | 0.072 ± 0.030 | 0.219 ± 0.091 | 1.315 ± 0.165 | 3.973 ± 0.291 | 0.200 ± 0.019 | 0.605 ± 0.028 |
| HFD | 0.530 ± 0.155 a | 1.059 ± 0.252 a | 1.572 ± 0.202 a | 3.182 ± 0.334 a | 0.237 ± 0.025 a | 0.486 ± 0.084 a |
| +Simvastatin | 0.189 ± 0.129 b,c | 0.464 ± 0.299 b,c | 1.266 ± 0.123 c | 3.198 ± 0.366 a | 0.223 ± 0.030 | 0.563 ± 0.076 |
| +Silymarin | 0.441 ± 0.291 a | 0.918 ± 0.508 a | 1.423 ± 0.174 | 3.259 ± 0.589 a | 0.220 ± 0.026 | 0.506 ± 0.111 b |
| +Metformin | 0.179 ± 0.104 b,c | 0.446 ± 0.256 b,c | 1.527 ± 0.146 b | 3.910 ± 0.402 c | 0.237 ± 0.031 a | 0.604 ± 0.051 c |
| +RG 250 mg/kg | 0.412 ± 0.251 b | 0.864 ± 0.463 b | 1.406 ± 0.156 | 3.226 ± 0.428 a | 0.249 ± 0.030 a | 0.517 ± 0.101 b |
| +fRG 500 mg/kg | 0.289 ± 0.234 d | 0.695 ± 0.507 b | 1.449 ± 0.116 | 3.869 ± 0.903 | 0.224 ± 0.016 | 0.602 ± 0.158 |
| +fRG 250 mg/kg | 0.308 ± 0.099 a,c | 0.691 ± 0.198 a,c | 1.435 ± 0.241 | 3.237 ± 0.293 a | 0.209 ± 0.030 d | 0.474 ± 0.061 a |
| +fRG 125 mg/kg | 0.308 ± 0.278 | 0.685 ± 0.605 | 1.389 ± 0.184 b | 3.360 ± 0.734 | 0.202 ± 0.032 d | 0.481 ± 0.066 a |
3.2. Effect of fRG on HFD-Induced Hyperglycemia

3.3. Effect of fRG on HFD-Induced Hyperlipidemia
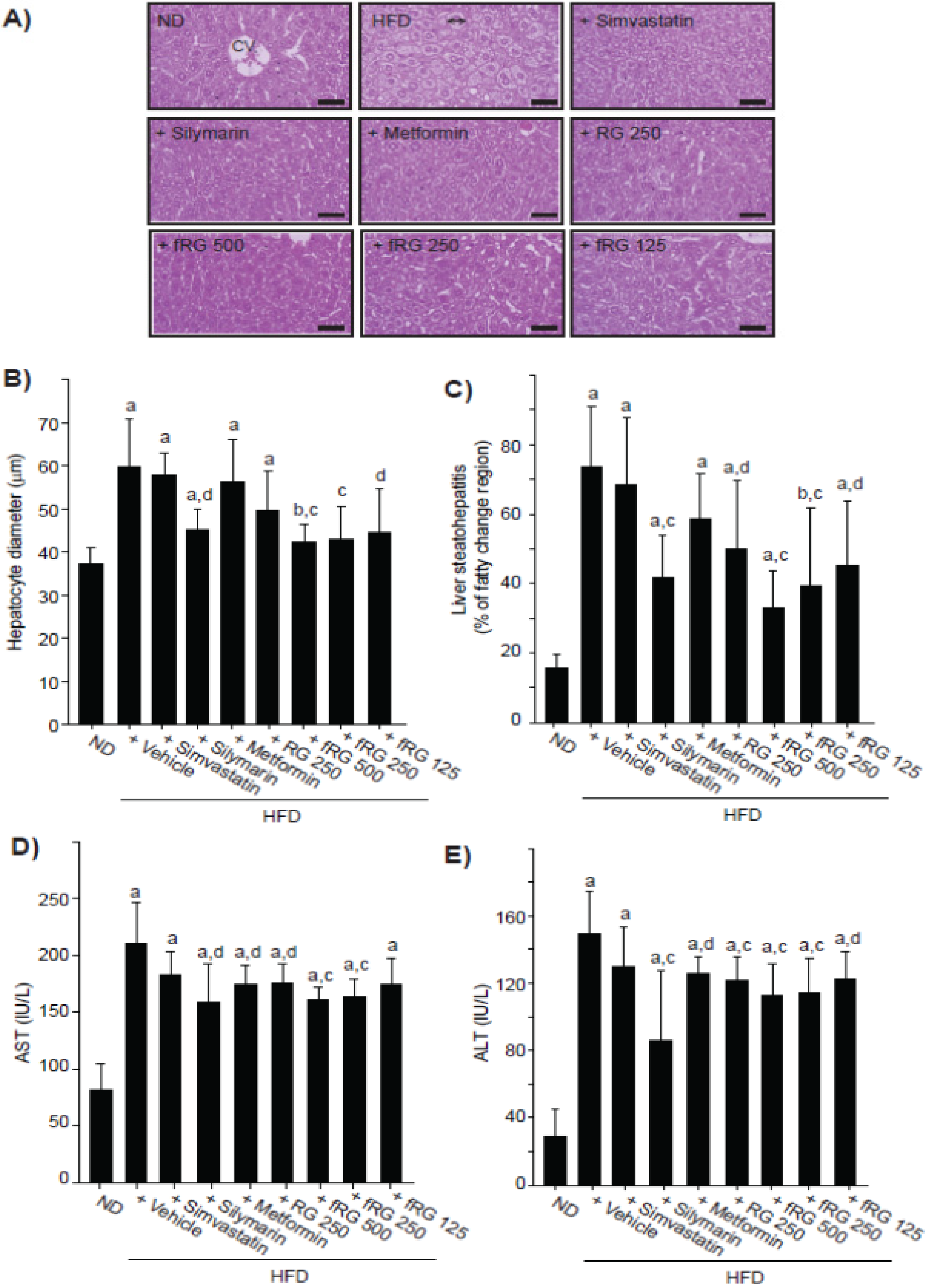
3.4. Effect of fRG on HFD-Induced Hepatopathy
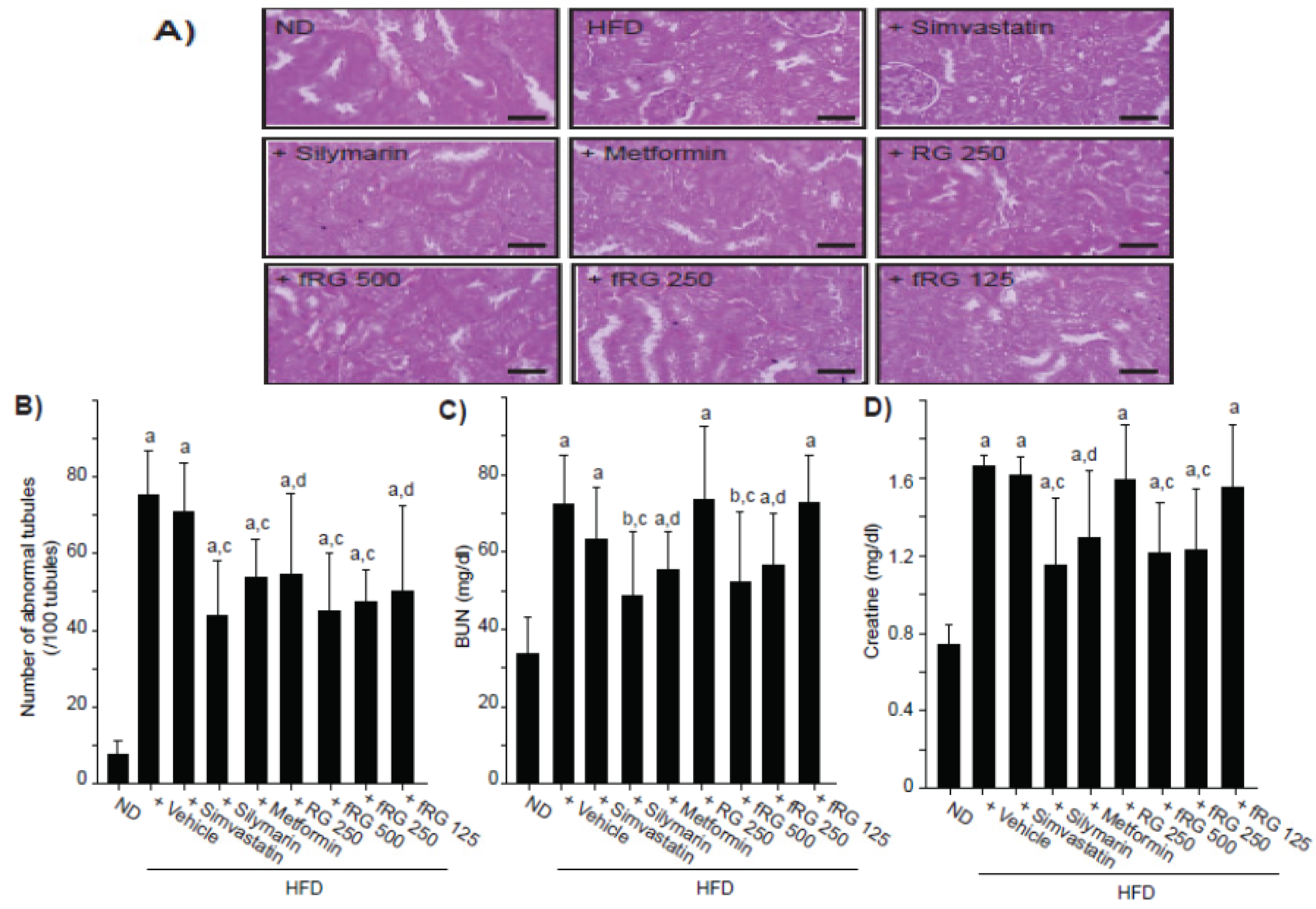
3.5. Effect of fRG on HFD-Induced Nephropathy
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mitchell, M.; Armstrong, D.T.; Robker, R.L.; Norman, R.J. Adipokines: Implications for female fertility and obesity. Reproduction 2005, 130, 583–597. [Google Scholar] [CrossRef]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef]
- Zalesin, K.C.; Franklin, B.A.; Miller, W.M.; Peterson, E.D.; McCullough, P.A. Impact of obesity on cardiovascular disease. Med. Clin. N. Am. 2011, 95, 919–937. [Google Scholar]
- Atshaves, B.P.; Martin, G.G.; Hostetler, H.A.; McIntosh, A.L.; Kier, A.B.; Schroeder, F. Liver fatty acid-binding protein and obesity. J. Nutr. Biochem. 2010, 21, 1015–1032. [Google Scholar]
- Guebre-Egziabher, F.; Alix, P.M.; Koppe, L.; Pelletier, C.C.; Kalbacher, E.; Fouque, D.; Soulage, C.O. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 2013, 95, 1971–1979. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unraveling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Sone, H.; Suzuki, H.; Takahashi, A.; Yamada, N. Disease model: Hyperinsulinemia and insulin resistance: Part A-targeted disruption of insulin signaling or glucose transport. Trends Mol. Med. 2001, 7, 320–322. [Google Scholar]
- Jung, Y.M.; Lee, S.H.; Lee, D.S.; You, M.J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.S.; Lee, Y.J.; Ku, S.K. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr. Res. 2011, 31, 387–396. [Google Scholar]
- Yun, S.N.; Moon, S.J.; Ko, S.K.; Im, B.O.; Chung, S.H. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch. Pharm. Res. 2004, 27, 790–796. [Google Scholar]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. JAMA 2002, 287, 360–372. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Kim, J.H. Cardiovascular diseases and Panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng Res. 2012, 36, 16–26. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, H.; Ye, J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 99–111. [Google Scholar]
- Jung, H.J.; Choi, H.; Lim, H.W.; Shin, D.; Kim, H.; Kwon, B.; Lee, J.E.; Park, E.H.; Lim, C.J. Enhancement of anti-inflammatory and antinociceptive actions of red ginseng extract by fermentation. J. Pharm. Pharmacol. 2012, 64, 756–762. [Google Scholar]
- Yun, S.N.; Ko, S.K.; Lee, K.H.; Chung, S.H. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch. Pharm. Res. 2007, 30, 587–595. [Google Scholar]
- Trinh, H.T.; Han, S.J.; Kim, S.W.; Lee, Y.C.; Kim, D.H. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J. Microbiol. Biotechnol. 2007, 17, 1127–1133. [Google Scholar]
- Yasuda, M.; Tachibana, S.; Kuba-Miyara, M. Biochemical aspects of red koji and tofuyo prepared using Monascus fungi. Appl. Microbiol. Biotechnol. 2012, 96, 49–60. [Google Scholar]
- Kim, H.Y.; Kang, K.S.; Yamabe, N.; Yokozawa, T. Comparison of the effects of Korean ginseng and heat-processed Korean ginseng on diabetic oxidative stress. Am. J. Chin. Med. 2008, 36, 989–1004. [Google Scholar] [CrossRef]
- Xie, J.T.; Wang, C.Z.; Ni, M.; Wu, J.A.; Mehendale, S.R.; Aung, H.H.; Foo, A.; Yuan, C.S. American ginseng berry juice intake reduces blood glucose and body weight in ob/ob mice. J. Food Sci. 2007, 72, S590–S594. [Google Scholar]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.J.; Zhao, L.C.; Zheng, Y.N.; Chen, L.; Yang, G.L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Hu, M.Y.; Guo, H.F.; Li, J.; Yu, Y.L.; Jin, S.; Wang, X.T.; Liu, L.; Liu, X.D. Increased glucagon-like peptide-1 secretion may be involved in antidiabetic effects of ginsenosides. J. Endocrinol. 2013, 217, 185–196. [Google Scholar]
- Dong, G.Z.; Jang, E.J.; Kang, S.H.; Cho, I.J.; Park, S.D.; Kim, S.C.; Kim, Y.W. Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement. Altern. Med. 2013, 13, 64. [Google Scholar]
- Lee, H.M.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Ginsenoside Rg1 promotes glucose uptake through activated AMPK pathway in insulin-resistant muscle cells. Phytother. Res. 2012, 26, 1017–1022. [Google Scholar]
- Lee, H.J.; Lee, Y.H.; Park, S.K.; Kang, E.S.; Kim, H.J.; Lee, Y.C.; Choi, C.S.; Park, S.E.; Ahn, C.W.; Cha, B.S.; et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism 2009, 58, 1170–1177. [Google Scholar]
- Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Kim, S.J.; Jung, M.S.; Chung, S.H. Ginsenoside Rg2 induces orphan nuclear receptor SHP gene expression and inactivates GSK3β via AMP-activated protein kinase to inhibit hepatic glucose production in HepG2 cells. Chem. Biol. Interact. 2012, 195, 35–42. [Google Scholar]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar]
- Ryu, J.S.; Lee, H.J.; Bae, S.H.; Kim, S.Y.; Park, Y.; Suh, H.J.; Jeong, Y.H. The bioavailability of red ginseng extract fermented by Phellinus linteus. J. Ginseng Res. 2013, 37, 108–116. [Google Scholar] [CrossRef]
- Lee, E.J.; Song, M.J.; Kwon, H.S.; Ji, G.E.; Sung, M.K. Oral administration of fermented red ginseng suppressed ovalbumin-induced allergic responses in female BALB/c mice. Phytomedicine 2012, 19, 896–903. [Google Scholar]
- Kim, H.J.; Lee, S.G.; Chae, I.G.; Kim, M.J.; Im, N.K.; Yu, M.H.; Lee, E.J.; Lee, I.S. Antioxidant effects of fermented red ginseng extracts in streptozotocin-induced diabetic rats. J. Ginseng Res. 2011, 35, 129–137. [Google Scholar]
- Morange, P.E.; Lijnen, H.R.; Alessi, M.C.; Kopp, F.; Collen, D.; Juhan-Vague, I. Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1150–1154. [Google Scholar]
- Park, S.H.; Ko, S.K.; Chung, S.H. Euonymus alatus prevents the hyperglycemia and hyperlipidemia induced by high-fat diet in ICR mice. J. Ethnopharmacol. 2005, 102, 326–335. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, Y.S.; Park, B.S.; Kim, J.E.; Lee, S.E. Hypolipidaemic effect of Hericium erinaceum grown in Artemisia capillaris on obese rats. Mycobiology 2013, 41, 94–99. [Google Scholar] [CrossRef]
- Hatzis, G.; Ziakas, P.; Kavantzas, N.; Triantafyllou, A.; Sigalas, P.; Andreadou, I.; Ioannidis, K.; Chatzis, S.; Filis, K.; Papalampros, A.; et al. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J. Hepatol. 2013, 5, 160–169. [Google Scholar]
- Seo, J.B.; Park, S.W.; Choe, S.S.; Jeong, H.W.; Park, J.Y.; Choi, E.W.; Seen, D.S.; Jeong, J.Y.; Lee, T.G. Foenumoside B from Lysimachia foenum-graecum inhibits adipocyte differentiation and obesity induced by high-fat diet. Biochem. Biophys. Res. Commun. 2012, 417, 800–806. [Google Scholar]
- Hoffler, U.; Hobbie, K.; Wilson, R.; Bai, R.; Rahman, A.; Malarkey, D.; Travlos, G.; Ghanayem, B.I. Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine 2009, 336, 311–325. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, C.M.; Yi, S.J.; Cho, I.J.; Ku, S.K. Red-Koji Fermented Red Ginseng Ameliorates High Fat Diet-Induced Metabolic Disorders in Mice. Nutrients 2013, 5, 4316-4332. https://doi.org/10.3390/nu5114316
Kim CM, Yi SJ, Cho IJ, Ku SK. Red-Koji Fermented Red Ginseng Ameliorates High Fat Diet-Induced Metabolic Disorders in Mice. Nutrients. 2013; 5(11):4316-4332. https://doi.org/10.3390/nu5114316
Chicago/Turabian StyleKim, Chang Man, Seong Joon Yi, Il Je Cho, and Sae Kwang Ku. 2013. "Red-Koji Fermented Red Ginseng Ameliorates High Fat Diet-Induced Metabolic Disorders in Mice" Nutrients 5, no. 11: 4316-4332. https://doi.org/10.3390/nu5114316




