Long-Term Consumption of Oats in Adult Celiac Disease Patients
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Dietary Assessment
2.3. Small-Bowel Mucosal Morphology and Inflammation
2.4. Gastrointestinal Symptoms and Clinical Evaluation
2.5. Serology and Chemical Analysis
2.6. Statistics
3. Results
| Characteristics | No Oats n = 36 | Oats n = 70 | |
|---|---|---|---|
| Female (%) | 25 (69%) | 46 (66%) | |
| Median age at time of study (range), years | 54 (36–73) | 59 (24–81) | |
| Symptoms and signs leading to the diagnosis of CD, n (%) | |||
| Abdominal symptoms | 29 (81%) | 61 (87%) | |
| Malabsorption, anemia, loss of weight | 17 (47%) | 47 (67%) | |
| Dermatitis herpetiformis | 4 (11%) | 9 (13%) | |
| Extraintestinal symptoms a | 5 (14%) | 11 (16%) | |
| Screening of risk groups b | 2 (6%) | 4 (6%) | |
| Family history of CD, n (%) | 10 (28%) | 42 (60%) c | |
| Median duration of gluten-free diet (range), years | 10 (1–28) | 8 (1–41) | |
| Median duration of oat consumption after the diagnosis of CD (range), years | 0 | 5 (0.5–8) | |
| Median (range) daily intake of oats, g | 0 | 20 (1–100) | |
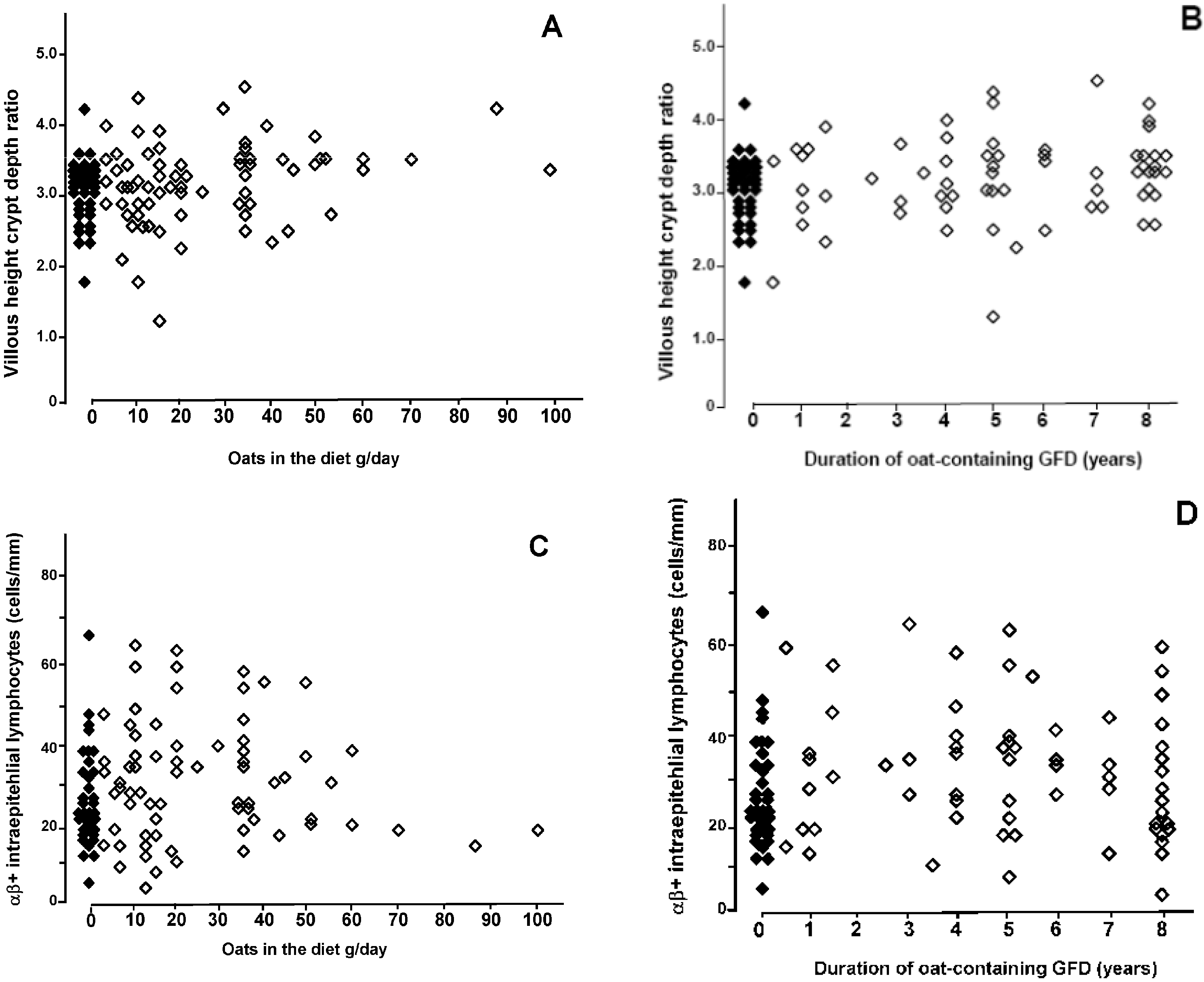
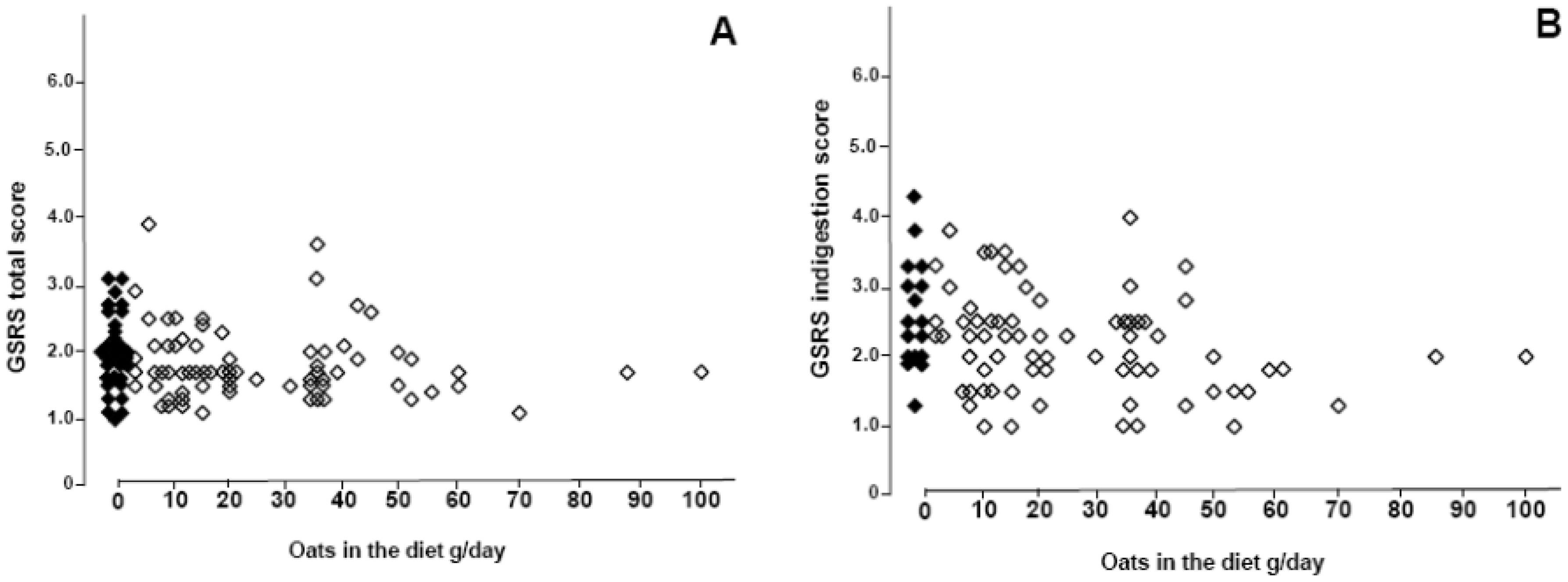
4. Discussion
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Storsrud, S.; Hulthen, L.R.; Lenner, R.A. Beneficial effects of oats in the gluten-free diet in adults with special reference to nutrient status, symptoms and subjective experiences. Br. J. Nutr. 2003, 90, 101–107. [Google Scholar] [CrossRef]
- Kemppainen, T.A.; Heikkinen, M.T.; Ristikankare, M.K.; Kosma, V.M.; Julkunen, R.J. Nutrient intakes during diets including unkilned and large amounts of oats in celiac disease. Eur. J. Clin. Nutr. 2010, 64, 62–67. [Google Scholar] [CrossRef]
- Lovik, A.; Gjoen, A.U.; Morkrid, L.; Guttormsen, V.; Ueland, T.; Lundin, K.E.A. Oats in a strictly gluten-free diet is associated with decreased gluten intake and increased serum bilirubin. e-SPEN 2009, 4, e315–e320. [Google Scholar] [CrossRef]
- Holm, K.; Mäki, M.; Vuolteenaho, N.; Mustalahti, K.; Ashorn, M.; Ruuska, T.; Kaukinen, K. Oats in the treatment of chilhood coeliac disease: A two-year controlled and a long-term clinical follow-up study. Aliment. Pharmacol. Ther. 2006, 23, 1463–1472. [Google Scholar] [CrossRef]
- Janatuinen, E.K.; Kemppainen, T.A.; Julkunen, R.J.K.; Kosma, V.-M.; Mäki, M.; Heikkinen, M.; Uusitupa, M.I.J. No harm from five year ingestion of oats in coeliac disease. Gut 2002, 50, 332–335. [Google Scholar] [CrossRef]
- Pulido, O.M.; Gillespie, Z.; Zarkadas, M.; Dubois, S.; Vavasour, E.; Rashid, M.; Switzer, C.; Godefroy, S.B. Introduction of oats in the diet of individuals with celiac disease: A systematic review. Adv. Food. Nutr. Res. 2009, 57, 235–285. [Google Scholar] [CrossRef]
- Sey, M.S.; Parfitt, J.; Gregor, J. Prospective study of clinical and histological safety of pure and uncontaminated Canadian oats in the management of celiac disease. JPEN 2011, 35, 459–464. [Google Scholar] [CrossRef]
- Butzner, J.D. Pure oats and the gluten-free diet: Are they safe? JPEN 2011, 35, 447–448. [Google Scholar] [CrossRef]
- Fric, P.; Gabrovska, D.; Nevoral, J. Celiac disease, gluten-free diet, and oats. Nutr. Rev. 2011, 69, 107–115. [Google Scholar] [CrossRef]
- Parakkal, D.V.; Du, H.; Semer, R.; Ehrenpreis, E.D.; Guandalini, S. Do gastroenterologists adhere to diagnostic and treatment guidelines for celiac disease? J. Clin. Gastroenterol. 2012, 46, e12–e20. [Google Scholar] [CrossRef]
- Hernando, A.; Mujico, J.R.; Juanas, D.; Mendez, E. Confirmation of the cereal type in oat products highly contaminated with gluten. J. Am. Diet. Assoc. 2006, 106, 665–666. [Google Scholar]
- Koerner, T.B.; Cléroux, C.; Poirier, C.; Cantin, I.; Alimkulov, A.; Elamparo, H. Gluten contamination in the Canadian commercial oat supply. Food Addit. Contam. 2011, 28, 705–710. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, O.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, e1. [Google Scholar] [CrossRef] [Green Version]
- Lundin, K.E.A.; Nilsen, E.M.; Scott, H.G.; Loberg, E.M.; Gjoen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Lovik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652. [Google Scholar]
- Vader, L.W.; Stepniak, D.T.; Bunnik, E.M.; Kooy, Y.M.C.; de Haan, W.; Drijfhout, J.W.; van Veelen, P.A.; Koning, F. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology 2003, 125, 1105–1113. [Google Scholar] [CrossRef]
- Hogberg, L.; Laurin, P.; Falth-Magnusson, K.; Grant, C.; Grodzinsky, E.; Jansson, G.; Ascher, H.; Browaldh, L.; Hammersjo, J.A.; Lindberg, E.; et al. Oats to children with newly diagnosed coeliac disease: A randomised double blind study. Gut 2004, 53, 649–654. [Google Scholar] [CrossRef]
- Peräaho, M.; Kaukinen, K.; Mustalahti, K.; Vuolteenaho, N.; Mäki, M.; Laippala, P.; Collin, P. Effect of an oats-containing gluten-free diet on symptoms and quality of life in coeliac disease. A randomized study. Scand. J. Gastroenterol. 2004, 39, 27–31. [Google Scholar]
- Peräaho, M.; Collin, P.; Kaukinen, K.; Kekkonen, L.; Miettinen, S.; Mäki, M. Oats can diversify a gluten-free diet in celiac disease and dermatitis herpetiformis. J. Am. Diet. Assoc. 2004, 104, 1148–1150. [Google Scholar] [CrossRef]
- Järvinen, T.T.; Kaukinen, K.; Laurila, K.; Kyrönpalo, S.; Rasmussen, M.; Mäki, M.; Korhonen, H.; Reunala, T.; Collin, P. Intraepithelial lymphocytes in celiac disease. Am. J. Gastroenterol. 2003, 98, 1332–1337. [Google Scholar] [CrossRef]
- Dimenäs, E.; Carlsson, H.; Glise, H.; Israelsson, B.; Wiklund, I. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand. J. Gastroenterol. 1996, 31, 8–13. [Google Scholar] [CrossRef]
- Midhagen, G.; Hallert, C. High rate of gastrointestinal symptoms in celiac patients living on a gluten-free diet: Controlled study. Am. J. Gastroenterol. 2003, 98, 2023–2026. [Google Scholar] [CrossRef]
- Mustalahti, K.; Lohiniemi, S.; Collin, P.; Vuolteenaho, N.; Laippala, P.; Mäki, M. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff. Clin. Pract. 2002, 5, 105–113. [Google Scholar]
- Mälkki, Y. Trends in dietary fibre research and development. Acta Aliment. 2004, 33, 39–62. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; de Lorenzo, L.; Cornell, H.; López-Casado, M.Á.; Barro, F.; Lorite, P.; Torres, M.I.; Cebolla, A.; Sousa, C. Diversity in oat potential immunogenicity: Basis for the selection of oat varieties with no toxicity in coeliac disease. Gut 2011, 60, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; de Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar]
- Collin, P.; Thorell, L.; Kaukinen, K.; Mäki, M. The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment. Pharmacol. Ther. 2004, 19, 1277–1283. [Google Scholar] [CrossRef]
- Kanerva, P.M.; Sontag-Strohm, T.S.; Ryöppy, P.H.; Alho-Lehto, P.; Salovaara, H.O. Analysis of barley contamination in oats using R5 and omega-gliadin antibodies. J. Cereal Sci. 2006, 44, 347–352. [Google Scholar] [CrossRef]
- Storsrud, S.; Malmheden Yman, I.; Lenner, R.A. Gluten contamination in oat products and products naturally free from gluten. Eur. Food Res. Technol. 2003, 217, 481–485. [Google Scholar] [CrossRef]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–308. [Google Scholar] [CrossRef]
- Lee, S.K.; Lo, W.; Memeo, L.; Rotterdam, H.; Green, P.H.R. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest. Endosc. 2003, 57, 187–191. [Google Scholar] [CrossRef]
- Ilus, T.; Lähdeaho, M.-L.; Salmi, T.; Haimila, K.; Partanen, J.; Saavalainen, P.; Huhtala, H.; Mäki, M.; Collin, P.; Kaukinen, K. Persistent duodenal intraepithelial lymphocytosis despite a long-term strict gluten-free diet in celiac disease. Am. J. Gastroenterol. 2012, 107, 1563–1569. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaukinen, K.; Collin, P.; Huhtala, H.; Mäki, M. Long-Term Consumption of Oats in Adult Celiac Disease Patients. Nutrients 2013, 5, 4380-4389. https://doi.org/10.3390/nu5114380
Kaukinen K, Collin P, Huhtala H, Mäki M. Long-Term Consumption of Oats in Adult Celiac Disease Patients. Nutrients. 2013; 5(11):4380-4389. https://doi.org/10.3390/nu5114380
Chicago/Turabian StyleKaukinen, Katri, Pekka Collin, Heini Huhtala, and Markku Mäki. 2013. "Long-Term Consumption of Oats in Adult Celiac Disease Patients" Nutrients 5, no. 11: 4380-4389. https://doi.org/10.3390/nu5114380




