Iron: Protector or Risk Factor for Cardiovascular Disease? Still Controversial
Abstract
:1. Introduction
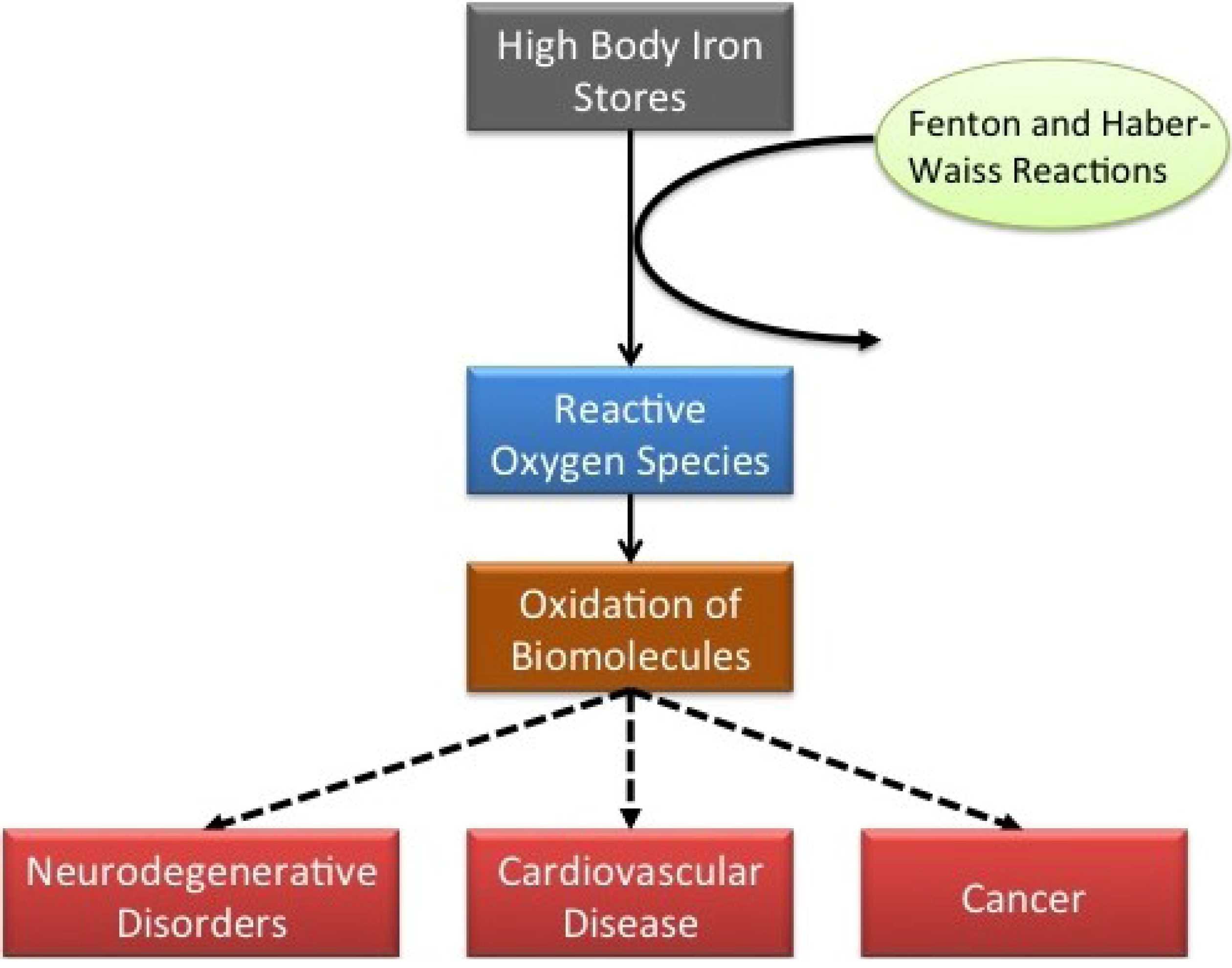
2. Studies Finding an Association between Iron and CVD
2.1. Cross-Sectional Studies
2.2. Case-Control Studies
2.3. Retrospective Cohort Study
2.4. Prospective Studies
2.5. Clinical Trials
3. Studies Which Did not Find Any Association between Iron and CVD
3.1. Cross-Sectional Study
3.2. Case-Control Study
3.3. Prospective Studies
3.4. Clinical Trial
3.5. Meta-Analysis
4. Conclusions
| STUDY DESING | AUTHOR | YEAR | REFERENCE |
|---|---|---|---|
| Cross-sectional | Kiechl et al. | 1994 | [2] |
| Haidari et al. | 2001 | [3] | |
| Wolff et al. | 2004 | [4] | |
| Zheng et al. | 2005 | [5] | |
| Menke et al. | 2009 | [6] | |
| Rajapurkar et al. | 2012 | [7] | |
| Syrovatka et al. | 2012 | [8] | |
| Sung et al. | 2012 | [9] | |
| Leiva et al. | 2013 | [10] | |
| Case-control | Toumainen et al. | 1998 | [12] |
| Klipstein-Gobrusch et al. | 1999 | [13] | |
| Meroño et al. | 2011 | [16] | |
| Holay et al. | 2012 | [17] | |
| Kang et al. | 2012 | [18] | |
| Retrospective Cohort | Meyers et al. | 2002 | [19] |
| Prospective Cohort | Salonen et al. | 1992 | [20] |
| Morrison et al. | 1994 | [21] | |
| Ascherio et al. | 1994 | [22] | |
| Magnusson et al. | 1994 | [25] | |
| Kiechl et al. | 1997 | [26] | |
| Salonen et al. | 1998 | [28] | |
| Van der A et al. | 2005 | [29] | |
| Zhang et al. | 2012 | [31] | |
| Clinical Trial | DePalma et al. | 2010 | [34] |
| Zacharski et al. | 2011 | [35] | |
| Houschyar et al. | 2012 | [38] | |
| DePalma et al. | 2013 | [36] |
| STUDY DESING | AUTHOR | YEAR | IRON AND CVD RELATIONSHIP | REFERENCE |
|---|---|---|---|---|
| Cross-sectional | Solymoss et al. | 1994 | None | [39] |
| Case-control | Regnström et al. | 1994 | Inverse | [11] |
| Mänttäri et al. | 1994 | None | [40] | |
| Moore et al. | 1995 | None | [41] | |
| Eichner et al. | 1998 | None | [42] | |
| Enbergs et al. | 1998 | None | [43] | |
| Tang et al. | 2003 | None | [44] | |
| Braun et al. | 2004 | None | [45] | |
| Kervinen et al. | 2004 | Inverse | [14] | |
| Ekblom et al. | 2011 | Inverse | [15] | |
| Shi et al. | 2011 | None | [46] | |
| Prospective Cohort | Baer et al. | 1994 | None | [47] |
| Sempos et al. | 1994 | Inverse | [23] | |
| Liao et al. | 1994 | Inverse | [24] | |
| Reunanen et al. | 1995 | None | [48] | |
| van Asperen et al. | 1995 | None | [49] | |
| Corti et al. | 1997 | Inverse | [27] | |
| Marniemi et al. | 1998 | None | [50] | |
| Manfroi et al. | 1999 | None | [51] | |
| Sempos et al. | 2000 | None | [52] | |
| Ascherio et al. | 2001 | None | [53] | |
| Knuiman et al. | 2003 | None | [54] | |
| van der A et al. | 2006 | Inverse | [30] | |
| Friedrich et al. | 2009 | None | [55] | |
| Menke et al. | 2012 | None | [57] | |
| Kim et al. | 2012 | Inverse | [32] | |
| Clinical Trial | Zacharski et al. | 2007 | None | [33] |
| Meta-analysis | Danesh et al. | 1999 | None | [58] |
Conflict of Interest
References
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [Google Scholar] [CrossRef]
- Kiechl, S.; Aichner, F.; Gerstenbrand, F.; Egger, G.; Mair, A.; Rungger, G.; Spögler, F.; Jarosch, E.; Oberhollenzer, F.; Willeit, J. Body iron stores and presence of carotid atherosclerosis. Results from the Bruneck Study. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 1625–1630. [Google Scholar] [CrossRef]
- Haidari, M.; Javadi, E.; Sanati, A.; Hajilooi, M.; Ghanbili, J. Association of increased ferritin with premature coronary stenosis in men. Clin. Chem. 2001, 47, 1666–1672. [Google Scholar]
- Wolff, B.; Völzke, H.; Lüdemann, J.; Robinson, D.; Vogelgesang, D.; Staudt, A.; Kessler, C.; Dahm, J.B.; John, U.; Felix, S.B. Association between high serum ferritin levels and carotid atherosclerosis in the study of health in Pomerania (SHIP). Stroke 2004, 35, 453–457. [Google Scholar] [CrossRef]
- Zheng, H.; Cable, R.; Spencer, B.; Votto, N.; Katz, S.D. Iron stores and vascular function in voluntary blood donors. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1577–1583. [Google Scholar] [CrossRef]
- Menke, A.; Fernández-Real, J.M.; Muntner, P.; Guallar, E. The association of biomarkers of iron status with peripheral arterial disease in US adults. BMC Cardiovasc. Disord. 2009, 9, 34. [Google Scholar]
- Rajapurkar, M.M.; Shah, S.V.; Lele, S.S.; Hegde, U.N.; Lensing, S.Y.; Gohel, K.; Mukhopadhyay, B.; Eigenbrodt, M.L. Association of catalytic iron with cardiovascular disease. Am. J. Cardiol. 2012, 109, 438–442. [Google Scholar] [CrossRef]
- Syrovatka, P.; Kraml, P.; Hulikova, K.; Fialova, L.; Vejrazka, M.; Crkovska, J.; Potockova, J.; Andel, M. Iron stores are associated with asymptomatic atherosclerosis in healthy men of primary prevention. Eur. J. Clin. Investig. 2011, 41, 846–853. [Google Scholar] [CrossRef]
- Sung, K.C.; Kang, S.M.; Cho, E.J.; Park, J.B.; Wild, S.H.; Byrne, C.D. Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2525–2530. [Google Scholar] [CrossRef]
- Leiva, E.; Mujica, V.; Sepúlveda, P.; Guzmán, L.; Núñez, S.; Orrego, R.; Palomo, I.; Andrews, M.; Arredondo, M.A. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol. Trace Elem. Res. 2013, 151, 1–8. [Google Scholar] [CrossRef]
- Regnström, J.; Tornvall, P.; Kallner, A.; Nilsson, J.; Hamsten, A. Stored iron levels and myocardial infarction at young age. Atherosclerosis 1994, 106, 123–125. [Google Scholar] [CrossRef]
- Tuomainen, T.P.; Punnonen, K.; Nyyssönen, K.; Salonen, J.T. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation 1998, 97, 1461–1466. [Google Scholar] [CrossRef]
- Klipstein-Grobusch, K.; Koster, J.F.; Grobbee, D.E.; Lindemans, J.; Boeing, H.; Hofman, A.; Witteman, J.C.M. Serum ferritin and risk of myocardial infarction in the elderly: The Rotterdam Study. Am. J. Clin. Nutr. 1999, 69, 1231–1236. [Google Scholar]
- Kervinen, H.; Tenkanen, L.; Palosuo, T.; Roivainen, M.; Manninen, V.; Mänttäri, M. Serum iron, infection and inflammation; effects on coronary risk. Scand. Cardiovasc. J. 2004, 38, 345–348. [Google Scholar] [CrossRef]
- Ekblom, K.; Marklund, S.L.; Jansson, J.H.; Hallmans, G.; Weinehall, L.; Hultdin, J. Iron stores and HFE genotypes are not related to increased risk of first-time myocardial infarction: A prospective nested case-referent study. Int. J. Cardiol. 2011, 150, 169–172. [Google Scholar] [CrossRef]
- Meroño, T.; Rosso, L.G.; Sorroche, P.; Boero, L.; Arbelbide, J.; Brites, F. High risk of cardiovascular disease in iron overload patients. Eur. J. Clin. Investig. 2011, 41, 479–486. [Google Scholar] [CrossRef]
- Holay, M.P.; Choudhary, A.A.; Suryawanshi, S.D. Serum ferritin—A novel risk factor in acute myocardial infarction. Indian Heart J. 2012, 64, 173–177. [Google Scholar]
- Kang, P.; Liu, T.; Tian, C.; Zhou, Y.; Jia, C. Association of total iron binding capacity with coronary artery disease. Clin. Chim. Acta 2012, 413, 1424–1429. [Google Scholar] [CrossRef]
- Meyers, D.G.; Jensen, K.C.; Menitove, J.E. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion 2002, 42, 1135–1139. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssonen, K.; Korpela, H.; Tuomilehto, J.; Seppanen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef]
- Morrison, H.I.; Semenciw, R.M.; Mao, Y.; Wigle, D.T. Serum iron and fatal acute myocardial infarction. Epidemiology 1994, 5, 243–246. [Google Scholar] [CrossRef]
- Ascherio, A; Willett, W.C.; Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J. Dietary iron intake and risk of coronary disease among men. Circulation 1994, 89, 969–974. [Google Scholar] [CrossRef]
- Sempos, C.T.; Looker, A.C.; Gillum, R.F.; Makuc, D.M. Body iron stores and the risk of coronary heart disease. N. Engl. J. Med. 1994, 330, 1119–1124. [Google Scholar]
- Liao, Y.; Cooper, R.S.; McGee, D.L. Iron status and coronary heart disease: Negative findings from the NHANES I epidemiologic follow-up study. Am. J. Epidemiol. 1994, 139, 704–712. [Google Scholar]
- Magnusson, M.K.; Sigfusson, N.; Sigvaldason, H.; Johannesson, G.M.; Magnusson, S.; Thorgeirsson, G. Low iron-binding capacity as a risk factor for myocardial infarction. Circulation 1994, 89, 102–108. [Google Scholar]
- Kiechl, S.; Willeit, J.; Egger, G.; Poewe, W.; Oberhollenzer, F. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the Bruneck study. Circulation 1997, 96, 3300–3307. [Google Scholar]
- Corti, M.C.; Guralnik, J.M.; Salive, M.E.; Ferrucci, L.; Pahor, M.; Wallace, R.B.; Hennekens, C.H. Serum iron level, coronary artery disease, and all-cause mortality in older men and women. Am. J. Cardiol. 1997, 79, 120–127. [Google Scholar] [CrossRef]
- Salonen, J.T.; Toumainem, T.P.; Salonen, R. Donation of blood is associated with reduced risk of myocardial infarction: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Epidemiol. 1998, 148, 445–451. [Google Scholar]
- Van der A, D.L.; Peeters, P.H.; Grobbee, D.E.; Marx, J.J.; van der Schouw, Y.T. Dietary haem iron and coronary heart disease in women. Eur. Heart J. 2005, 26, 257–262. [Google Scholar]
- Van der A, D.L.; Marx, J.J.; Grobbee, D.E.; Kamphuis, M.H.; Georgiou, N.A.; van Kats-Renaud, J.H.; Breuer, W.; Cabantchik, Z.I.; Roest, M.; Voorbij, H.A.M.; et al. Non-transferrin-bound iron and risk of coronary heart disease in postmenopausal women. Circulation 2006, 113, 1942–1949. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Ohira, T.; Date, O.C.; Tanabe, N.; Kikuchi, S.; Tamakoshi, A. Associations of dietary iron intake with mortality from cardiovascular disease: The JACC study. J. Epidemiol. 2012, 22, 484–493. [Google Scholar]
- Kim, K.S.; Son, H.G.; Hong, N.S.; Lee, D.H. Associations of serum ferritin and transferrin % saturation with all-cause, cancer, and cardiovascular disease mortality: Third National Health and Nutrition Examination Survey follow-up study. J. Prev. Med. Public Health 2012, 45, 196–203. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: A randomized controlled trial. JAMA 2007, 297, 603–610. [Google Scholar] [CrossRef]
- DePalma, R.G.; Hayes, V.W.; Chow, B.K.; Shamayeva, G.; May, P.E.; Zacharski, L.R. Ferritin levels, inflammatory biomarkers, and mortality in peripheral arterial disease: A substudy of the Iron (Fe) and Atherosclerosis Study (FeAST) Trial. J. Vasc. Surg. 2010, 51, 1498–1503. [Google Scholar]
- Zacharski, L.R.; Shamayeva, G.; Chow, B.K. Effect of controlled reduction of body iron stores on clinical outcomes in peripheral arterial disease. Am. Heart J. 2011, 162, 949–957. [Google Scholar] [CrossRef]
- DePalma, R.G.; Zacharski, L.R.; Chow, B.K.; Shamayeva, G.; Hayes, V.W. Reduction of iron stores and clinical outcomes in peripheral arterial disease: Outcome comparisons in smokers and non-smokers. Vascular 2013. [Google Scholar] [CrossRef]
- Zacharski, L.R.; DePalma, R.G.; Shamayeva, G.; Chow, B.K. The statin-iron nexus: Anti-inflammatory intervention for arterial disease prevention. Am. J. Public Health 2013, 103, 105–112. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Lüdtke, R.; Dobos, G.J.; Kalus, U.; Broecker-Preuss, M.; Rampp, T.; Brinkhaus, B.; Michalsen, A. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: Results from a randomized clinical trial. BMC Med. 2012, 10, 54. [Google Scholar] [CrossRef]
- Solymoss, B.C.; Marcil, M.; Gilfix, B.M.; Gelinas, F.; Poitras, A.M.; Campeau, L. The place of ferritin among risk factors associated with coronary artery disease. Coron. Artery Dis. 1994, 5, 231–235. [Google Scholar] [CrossRef]
- Mänttäri, M.; Manninen, V.; Huttunen, J.K.; Palosuo, T.; Ehnholm, C.; Heinonen, O.P.; Frick, M.H. Serum ferritin and ceruloplasmin as coronary risk factors. Eur. Heart J. 1994, 15, 1599–1603. [Google Scholar]
- Moore, M.; Folsom, A.R.; Barnes, R.W.; Eckfeldt, J.H. No association between serum ferritin and asymptomatic carotid atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol 1995, 141, 719–723. [Google Scholar]
- Eichner, J.E.; Qi, H.; Moore, W.E.; Schechter, E. Iron measures in coronary angiography patients. Atherosclerosis 1998, 136, 241–245. [Google Scholar] [CrossRef]
- Enbergs, A.; Dorszewski, A.; Luft, M.; Mönnig, G.; Kleemann, A.; Schulte, H.; Assmann, G.; Breithardt, G.; Kerber, S. Failure to confirm ferritin and caeruloplasmin as risk factors for the angiographic extent of coronary arteriosclerosis. Coron. Artery Dis. 1998, 9, 119–124. [Google Scholar]
- Tang, Y.R.; Zhang, S.Q.; Xiong, Y.; Zhao, Y.; Fu, H.; Zhang, H.P.; Xiong, K.M. Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart diseas. Biol. Trace Elem. Res. 2003, 92, 97–104. [Google Scholar] [CrossRef]
- Braun, S.; Ndrepepa, G.; von Beckerath, N.; Vogt, W.; Schömig, A.; Kastrati, A. Value of serum ferritin and soluble transferrin receptor for prediction of coronary artery disease and its clinical presentations. Atherosclerosis 2004, 174, 105–110. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, L.; Huang, L.H.; Lian, Y.T.; Zhang, X.M.; Gou, H.; Wu, T.C.; Cheng, L.X.; He, M.A. Plasma ferritin levels, genetic variations in HFE gene, and coronary heart disease in Chinese: A case-control study. Atherosclerosis 2011, 218, 386–390. [Google Scholar] [CrossRef]
- Baer, D.M.; Tekawa, I.S.; Hurley, L.B. Iron stores are not associated with acute myocardial infarction. Circulation 1994, 89, 2915–2918. [Google Scholar] [CrossRef]
- Reunanen, A.; Takkunen, H.; Knekt, P.; Seppänen, R.; Aromaa, A. Body iron stores, dietary iron intake and coronary heart disease mortality. J. Intern. Med. 1995, 238, 223–230. [Google Scholar] [CrossRef]
- Van Asperen, I.A.; Feskens, E.J.; Bowles, C.H.; Kromhout, D. Body iron stores and mortality due to cancer and ischaemic heart disease: A 17-year follow-up study of elderly men and women. Int. J. Epidemiol. 1995, 24, 665–670. [Google Scholar] [CrossRef]
- Marniemi, J.; Järvisalo, J.; Toikka, T.; Räihä, I.; Ahotupa, M.; Sourander, L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int. J. Epidemiol. 1998, 27, 799–807. [Google Scholar] [CrossRef]
- Manfroi, W.C.; Zago, A.J.; Caramori, P.R.; Cruz, R.; Oliveira, J.; Kirschnick, L.S.; Ordovás, K.; Candiago, R.H.; de Souza, J.; Ribeiro, L.W.; et al. Does serum ferritin correlate with coronary angiography findings? Int. J. Cardiol. 1999, 69, 149–153. [Google Scholar] [CrossRef]
- Sempos, C.T.; Looker, A.C.; Gillum, R.E.; McGee, D.L.; Vuong, C.V.; Johnson, C.L. Serum ferritin and death from all causes and cardiovascular disease: The NHANES II Mortality Study. National Health and Nutrition Examination Study. Ann. Epidemiol. 2000, 10, 441–448. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.; Willett, W.C.; Stampfer, M.J. Blood donations and risk of coronary heart disease in men. Circulation 2001, 103, 52–57. [Google Scholar] [CrossRef]
- Knuiman, M.W.; Divitini, M.L.; Olynyk, J.K.; Cullen, D.J.; Bartholomew, H.C. Serum ferritin and cardiovascular disease: A 17-year follow-up study in Busselton, Western Australia. Am. J. Epidemiol. 2003, 158, 144–149. [Google Scholar] [CrossRef]
- Friedrich, N.; Milman, N.; Völzke, H.; Linneberg, A.; Jørgensen, T. Is serum ferritin within the reference range a risk predictor of cardiovascular disease? A population-based, long-term study comprising 2874 subjects. Br. J. Nutr. 2009, 102, 594–600. [Google Scholar] [CrossRef]
- Sullivan, J.L. Misconceptions in the debate on the iron hypothesis. J. Nutr. Biochem. 2001, 12, 33–37. [Google Scholar] [CrossRef]
- Menke, A.; Muntner, P.; Fernández-Real, J.M.; Guallar, E. The association of biomarkers of iron status with mortality in US adults. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 734–740. [Google Scholar] [CrossRef]
- Danesh, J.; Appleby, P. Coronary heart disease and iron status: Meta-analyses of prospective studies. Circulation 1999, 99, 852–854. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Muñoz-Bravo, C.; Gutiérrez-Bedmar, M.; Gómez-Aracena, J.; García-Rodríguez, A.; Navajas, J.F.-C. Iron: Protector or Risk Factor for Cardiovascular Disease? Still Controversial. Nutrients 2013, 5, 2384-2404. https://doi.org/10.3390/nu5072384
Muñoz-Bravo C, Gutiérrez-Bedmar M, Gómez-Aracena J, García-Rodríguez A, Navajas JF-C. Iron: Protector or Risk Factor for Cardiovascular Disease? Still Controversial. Nutrients. 2013; 5(7):2384-2404. https://doi.org/10.3390/nu5072384
Chicago/Turabian StyleMuñoz-Bravo, Carlos, Mario Gutiérrez-Bedmar, Jorge Gómez-Aracena, Antonio García-Rodríguez, and Joaquín Fernández-Crehuet Navajas. 2013. "Iron: Protector or Risk Factor for Cardiovascular Disease? Still Controversial" Nutrients 5, no. 7: 2384-2404. https://doi.org/10.3390/nu5072384





