Eye Nutrition in Context: Mechanisms, Implementation, and Future Directions
Abstract
:1. Introduction
2. Colors and Nomenclature of Carotenoids
3. Carotenoids in the Functioning and Protection of the Human Eye
4. Dietary Sources of Zeaxanthin & Lutein
4.1. Carotenoid and Vitamin Supplements
4.2. Yellow Foods
4.3. Leafy Greens and Other Plant Sources
5. Xanthophylls in the Context of Other Dietary Modulators
5.1. Foods Contain Multiple Gene Regulators Acting in Synergy
5.2. Gene Regulators Produced by Lipid Peroxidation
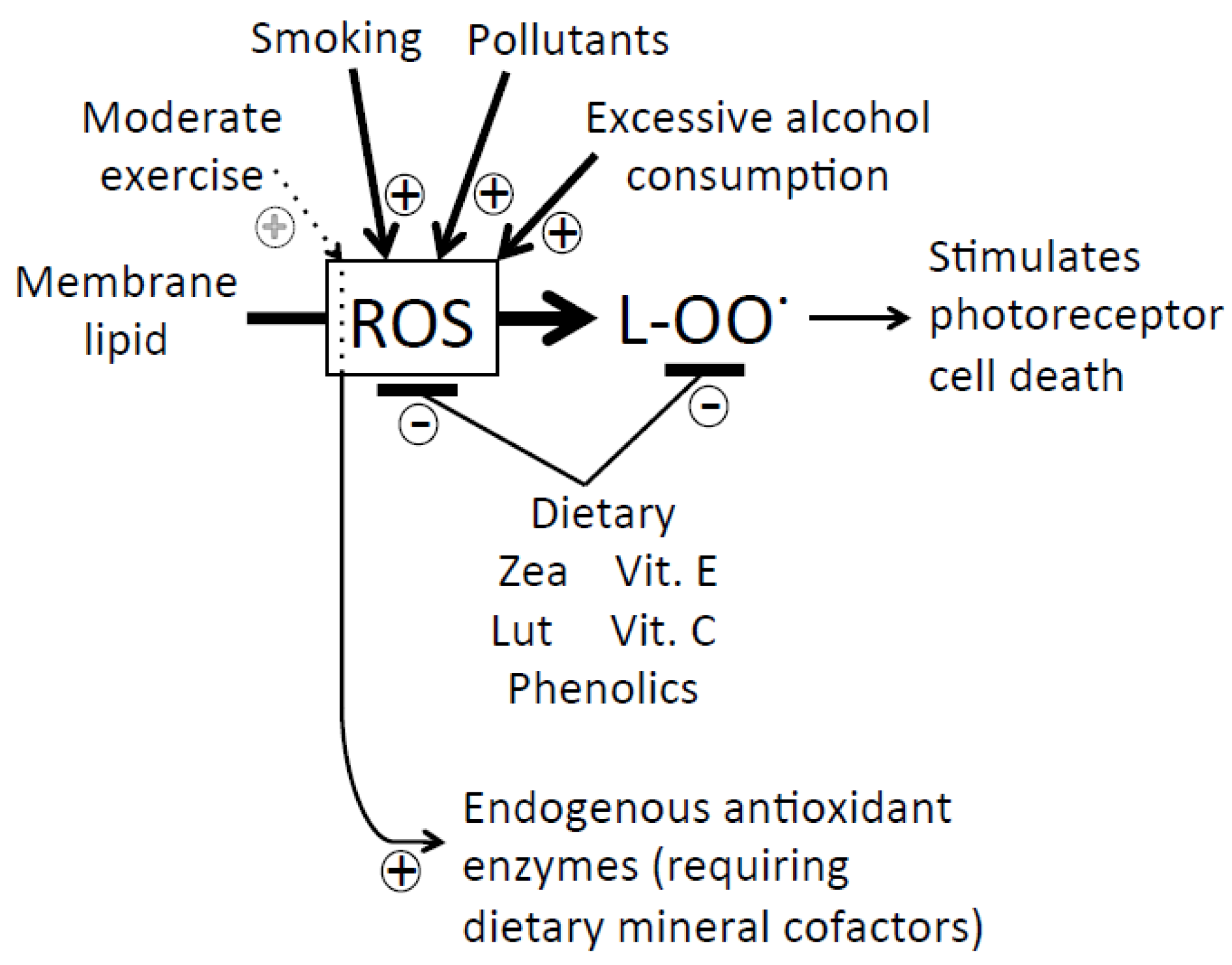
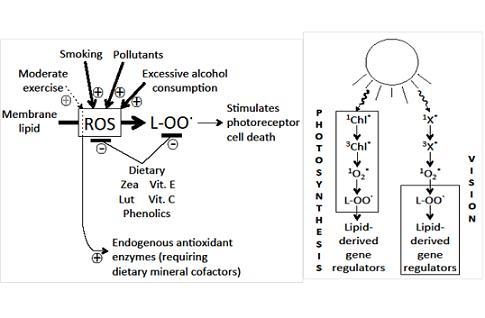
5.3. Poly-Unsaturated Fatty Acids
5.4. Dietary and Lifestyle Factors Increasing Disease Risk
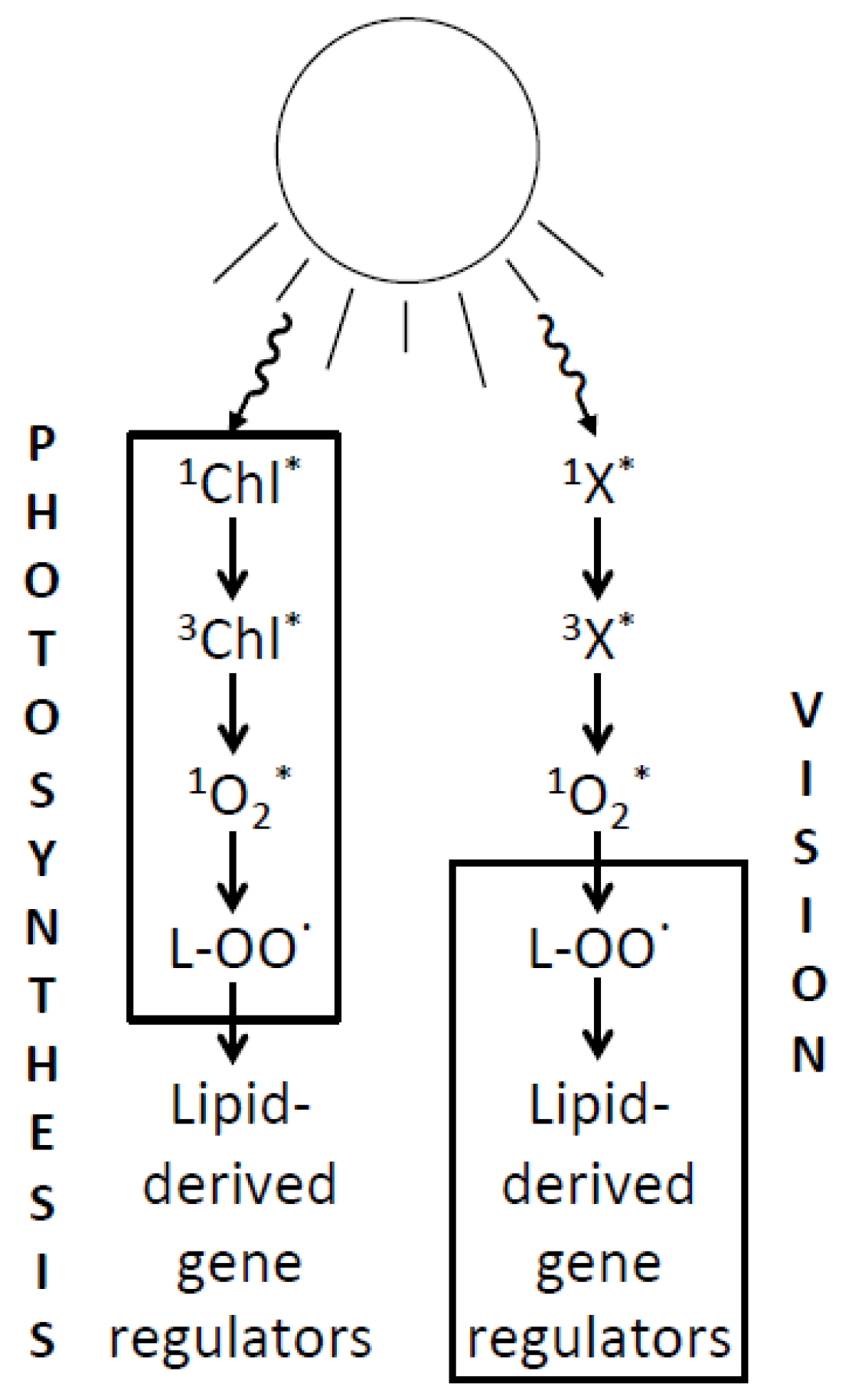
6. Relating the Mechanisms of Photoprotection to the Photochemistry of Photosynthesis and Human Vision
Acknowledgments
Conflict of Interest
References
- Demmig-Adams, B.; Rixham, C.S.; Adams, W.W., III. Carotenoids. In McGraw-Hill Encyclopedia of Science & Technology, 11th ed; McGraw-Hill: New York, NY, USA, 2012; pp. 549–555. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III. Overview of diet-gene interaction and the example of xanthophylls. Adv. Exp. Med. Biol. 2010, 698, 17–26. [Google Scholar] [CrossRef]
- Richer, S.B.; Stiles, W.; Graham-Hoffman, K.; Levin, M.; Ruskin, D.; Wrobel, J.; Park, D.W.; Thomas, C. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration. The zeaxanthin and visual function study (ZVF) FDA IND #78, 973. Optometry 2011, 82, 667–680. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Neuringer, M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: Promise of molecular genetics for guiding mechanistic and translational research in the field. Am. J. Clin. Nutr. 2012, 96, 1223S–1233S. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J. Nutr. 2002, 132, 518S–524S. [Google Scholar]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment zeaxanthin—A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Sabour-Pickett, S.; Nolan, J.M.; Loughman, J.; Beatty, S. A review of the evidence germane to the putative protective role of the macular carotenoids for the age-related macular degeneration. Mol. Nutr. Food Res. 2012, 56, 270–286. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M. Influence of the dietary carotenoids lutein and zeaxanthin on visual performance: Application to baseball. Am. J. Clin. Nutr. 2012, 96, 1207S–1213S. [Google Scholar] [CrossRef]
- Thomson, L.R.; Toyoda, Y.; Langner, A.; Delori, F.C.; Garnett, K.M.; Craft, N.E.; Nichols, C.R.; Cheng, K.M.; Dorey, C.K. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3538–3549. [Google Scholar]
- Thomson, L.R.; Toyoda, Y.; Delori, F.C.; Garnett, K.M.; Wong, Z.Y.; Nichols, C.R.; Cheng, K.M.; Craft, N.E.; Dorey, C.K. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp. Eye Res. 2002, 75, 529–542. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Mirowaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Chew, B.P.; Brown, C.M.; Park, J.S.; Mixter, P.F. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res. 2003, 23, 3333–3339. [Google Scholar]
- Sumatran, V.N.; Zhang, R.; Lee, D.S.; Wicha, M.S. Differential regulation of apoptosis in normal versus transformed mammary epithelium by lutein and retinoic acid. Cancer Epidemiol. Biomakers Prev. 2000, 9, 257–263. [Google Scholar]
- Müller, K.; Carpenter, K.L.H.; Challis, I.R.; Skepper, J.N.; Arends, M.J. Carotenoids induce apoptosis in the T-lymphoblast cell line Jurkat E6.1. Free Radic. Res. 2002, 36, 791–802. [Google Scholar]
- Maccarrone, M.; Bari, M.; Gasperi, V.; Demmig-Adams, B. The photoreceptor protector zeaxanthin induces cell death in neuroblastoma cells. Anticancer Res. 2005, 25, 3871–3876. [Google Scholar]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2012, 56, 228–240. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fuld, K.; Snodderly, D.M. Iris color and macular pigment optical density. Exp. Eye Res. 1996, 62, 293–297. [Google Scholar] [CrossRef]
- Tran, E.; Demmig-Adams, B. Vitamins and minerals: Powerful medicine or potent toxins? Nutr. Food Sci. 2007, 37, 50–60. [Google Scholar] [CrossRef]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar]
- Daicker, B.; Schiedt, K.; Adnet, J.J.; Bermond, P. Canthaxanthin retinophathy-an investigation by light and electron-microscopy and physicochemical analysis. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 189–197. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhofer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef]
- Ma, L.; Dou, H.L; Huang, Y.M.; Lu, X.R.; Qian, F.; Zou, Z.Y.; Pang, H.L.; Dong, P.C.; Xiao, X.; Wang, X.; et al. Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: A randomized, double-masked, placebo-controlled trial. Am. J. Ophthalmol. 154, 2012, 625–634. [Google Scholar]
- Bartlett, H.E.; Eperjesi, F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: A randomized controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1121–1127. [Google Scholar] [CrossRef]
- Cutler, R.G.; Plummer, J.; Chowdury, K.; Heward, C. Oxidative stress profiling Part II. Theory, technology, and practice. Ann. N. Y. Acad. Sci. 2005, 1055, 136–158. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar]
- Naviaux, R.K. Oxidative shielding or oxidative stress? J. Pharmcol. Exp. Ther. 2012, 342, 608–618. [Google Scholar] [CrossRef]
- De Oliveira, G.P.R.; Rodriguez-Amaya, D.B. Processed and prepared corn products as sources of lutein and zeaxanthin: Compositional variation in the food chain. J. Food Sci. 2007, 72, S079–S085. [Google Scholar] [CrossRef]
- Pintea, A.; Dulf, F.V.; Bunea, A.; Matea, C.; Andrei, S. Comparative analysis of lipophilic compounds in eggs of organically raised ISA Brown and Araucana hens. Chem. Pap. 2012, 66, 955–963. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W., III. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef]
- Pogson, B.J.; Nigoyi, K.K.; Björkman, O.; DellaPenna, D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc. Natl. Acad. Sci. USA 1998, 95, 13324–13329. [Google Scholar]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Cuine, S.; Guiliano, G.; Bassi, R. The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 13878–13888. [Google Scholar]
- Demmig-Adams, B. Linking the xanthophyll cycle with photoprotective energy dissipation. Photosynth. Res. 2003, 76, 73–80. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Dharmapuri, S.; Rosati, C.; Pallara, P.; Aquilani, R.; Bouvier, F.; Camara, B.; Guiliano, G. Metabolic engineering of xanthophyll content in tomato fruits. FEBS Lett. 2002, 519, 30–34. [Google Scholar] [CrossRef]
- Romer, S.; Lubeck, J.; Kauder, F.; Steiger, S.; Adomat, C.; Sandmann, G. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and cosuppression of carotenoid epoxidation. Metab. Eng. 2002, 4, 263–272. [Google Scholar]
- Albrecht, M.; Misawa, N.; Sandmann, G. Metabolic engineering of the terpenoid biosynthetic pathway of Escherichia coli for production of the carotenoids beta-carotene and zeaxanthin. Biotechnol. Lett. 1999, 21, 791–795. [Google Scholar] [CrossRef]
- Sin, H.P.Y.; Liu, D.T.L.; Lam, D.S.C. Lifestyle modification, nutritional and vitamin supplements for age-related macular degeneration. Acta Ophthalmol. 2013, 91, 6–11. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar]
- Lapillonne, A.; Clarke, S.D.; Heird, W.C. Polyunsaturated fatty acids and gene expression. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 151–156. [Google Scholar]
- Lavrovsky, Y.; Chatterjee, B.; Clark, R.A.; Roy, A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000, 35, 521–532. [Google Scholar]
- Seo, T.; Blaner, W.S.; Deckelbaum, R.J. Omega-3 fatty acids: Molecular approaches to optimal biological outcomes. Curr. Opin. Lipidol. 2005, 16, 11–18. [Google Scholar]
- Youdim, K.A.; Spencer, J.P.E.; Schroeter, H.; Rice-Evans, C. Dietary flavonoids as potential neuroprotectants. Biol. Chem. 2002, 383, 503–519. [Google Scholar]
- Maccarrone, M.; Melino, G.; Finazzi-Agrò, A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 2001, 8, 776–784. [Google Scholar]
- Tang, D.G.; La, E.; Kern, J.; Kehrer, J.P. Fatty acid oxidation and signaling in apoptosis. Biol. Chem. 2002, 383, 425–442. [Google Scholar]
- Lebeau, A.; Terro, F.; Rostene, W; Pelaprat, D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004, 11, 875–884. [Google Scholar]
- Grichenko, O.E.; Shaposhnikova, V.V.; Kudryavtsev, A.A.; Korystov, Y.N. Apoptosis in p388 leukemia cells induced by specific inhibitors of 5- and 12-lipoxygnease and the product of cyclooxygenase, prostaglandin E-2. Biol. Bull. 2004, 31, 221–225. [Google Scholar]
- Maccarrone, M.; Corasantini, M.T.; Guerrieri, P.; Nistico, G.; Finazzi-Agrò, A. Nitric oxide-donor compounds inhibit lipoxygenase activity. Biochem. Biophys. Res. Commun. 1996, 219, 128–133. [Google Scholar]
- Maccarrone, M.; Lorenzon, T.; Guerrieri, P.; Finazzi-Agrò, A. Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur. J. Biochem. 1999, 265, 27–34. [Google Scholar]
- Chitchumroonchokchai, C.; Bomser, J.A.; Glamm, J.E.; Failla, M.L. Xanthophylls and alpha-tocopherol decrease UVB-induced lipid peroxidation and stress signalling in human lens epithelial cells. J. Nutr. 2004, 134, 3225–3232. [Google Scholar]
- Wrona, M.; Korytowksi, W.; Ròzanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of antioxidants in protection against photosensitized oxidation. Free Radic. Biol. Med. 2003, 35, 1319–1329. [Google Scholar]
- Wrona, M.; Ròzanowska, M.; Sarna, T. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects APRE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar]
- Bartlett, H.; Eperjesi, F. An ideal ocular nutritional supplement? Ophthalmic Physiol. Opt. 2004, 24, 339–349. [Google Scholar]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acid ratio and chronic disease. Food Rev. Int. 2004, 20, 77–90. [Google Scholar]
- Haag, M.; Dippenaar, N.G. Dietary fats, fatty acids and insulin resistance: Short review of a multifaceted connection. Med. Sci. Monit. 2005, 11, RA359–RA367. [Google Scholar]
- Blaschke, F.; Takata, Y.; Caglayan, E.; Law, R.E.; Hsueh, W.A. Obesity, peroxisome proliferator-activated receptor and atherosclerosis in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 28–40. [Google Scholar] [CrossRef]
- Richardson, A.J.; Ross, M.A. Fatty acid metabolism in neurodevelopmental disorder: A new perspective on associations between attention-deficit/hyperactivity disorder, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 1–9. [Google Scholar] [CrossRef]
- Young, G.; Conquer, J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod. Nutr. Dev. 2004, 45, 1–28. [Google Scholar] [CrossRef]
- Wainwright, P.E. Dietary essential fatty acids and brain function: A developmental perspective on mechanisms. Proc. Nutr. Soc. 2002, 61, 61–69. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and cancer. Indoor Built Environ. 2003, 12, 405–412. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio, genetic variation and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17, 131–134. [Google Scholar]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar]
- Roncone, M.; Bartlett, H.; Eperjesi, F. Essential fatty acids for dry eye: A review. Cont. Lens Anterior Eye 2010, 33, 49–54. [Google Scholar] [CrossRef]
- Wisniewska-Becker, A.; Nawrocki, G.; Duda, M.; Subczynski, W.K. Structural aspects of the antioxidant activity of lutein in a model of photoreceptor membranes. Acta Biochim. Pol. 2012, 59, 119–123. [Google Scholar]
- Warner, K.; Knowlton, S. Frying quality and oxidative stability of high-oleic corn oils. J. Am. Oil Chem. Soc. 1997, 74, 1317–1322. [Google Scholar] [CrossRef]
- Forster, V.A. Genetically modified crop approvals and planted acreages. Crop Biotechnol. 2002, 829, 17–22. [Google Scholar] [CrossRef]
- Liu, Q.; Singh, S.P.; Green, A.G. High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated posttranscriptional gene silencing. Plant Physiol. 2002, 129, 1732–1743. [Google Scholar] [CrossRef]
- Liu, Q.; Singh, S.; Green, A. High-oleic and high-stearic cottonseed oils: Nutritionally improved cooking oils developed using gene silencing. J. Am. Coll. Nutr. 2002, 21, 205S–211S. [Google Scholar] [CrossRef]
- Smith, S.A.; King, R.E.; Min, D.B. Oxidative and thermal stabilities of genetically modified high oleic sunflower oil. Food Chem. 2007, 102, 1208–1213. [Google Scholar] [CrossRef]
- Herder, R.; Demmig-Adams, B. The power of a balanced diet and lifestyle in preventing cardiovascular disease. Nutr. Clin. Care 2004, 7, 46–55. [Google Scholar]
- Seddon, J.M. Multivitamin-multimineral supplements and eye disease: Age-related macular degeneration and cataract. Am. J. Clin. Nutr. 2007, 85, 304S–307S. [Google Scholar]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2012, 11, CD000254. [Google Scholar]
- Rozanowska, M.; Sarna, T. Light-induced damage to the retina: Role of the rhodopsin chromophore revisited. Photochem. Photobiol. 2005, 81, 1305–1330. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; University of Colorado, Boulder, CO, USA. Unpublished work, 2013.
- Hunter, J.J.; Morgan, J.I.W.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef]
- Rozanowska, M. Light-induced damage to the retina: current understanding of the mechanisms and unresolved questions: A symposium-in-print introduction. Photochem. Photobiol. 2012, 88, 1303–1308. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughn, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Glaeser, J.; Nuss, A.M.; Berghoff, B.A.; Klug, G. Singlet oxygen stress in microorganisms. Adv. Microb. Physiol. 2011, 58, 141–173. [Google Scholar] [CrossRef]
- Kim, S.R.; Nakanishi, K.; Itagaki, Y.; Sparrow, J.R. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp. Eye Res. 2006, 82, 828–839. [Google Scholar] [CrossRef]
- Loginova, M.Y.; Rostovtseva, Y.V.; Feldman, T.B.; Ostrovsky, M.A. Light damaging action of all-trans-retinal and its derivatives on rhodopsin molecules in the photoreceptor membrane. Biochemistry (Moscow) 2008, 73, 130–138. [Google Scholar] [CrossRef]
- Bhosale, P.; Serban, B.; Berstein, P.S. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch. Biochem. Biophys. 2009, 483, 175–181. [Google Scholar] [CrossRef]
- Maeda, T.; Golczak, M.; Maeda, A. Retinal photodamage mediated by all-trans-retinal. Photochem. Photobiol. 2012, 88, 1309–1319. [Google Scholar] [CrossRef]
- Masutomi, K.; Chen, C.H.; Nakatani, K.; Koutalos, Y. All-trans retinal mediates light-induced oxidation in single living rod photoreceptors. Photochem. Photobiol. 2012, 88, 1356–1361. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- Betterle, N.; Ballottari, M.; Hienerwadel, R.; Dall’Osto, L.; Bassi, R. Dynamics of zeaxanthin binding to the photosystem II monomeric antenna protein Lhcb6 (CP24) and modulation of its photoprotection properties. Arch. Biochem. Biophys. 2010, 504, 67–77. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Amiard, V.; van Zadelhoff, G.; Veldink, G.A.; Muller, O.; Adams, W.W., III. Emerging trade-offs—Impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins as regulators of development and defense. New Phytol. 2013, 197, 720–729. [Google Scholar] [CrossRef]
- Li, B.X.; Ahmed, F.; Berstein, P.S. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch. Biochem. Biophys. 2010, 504, 56–60. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P.; Duffy, C.D.P. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta 2012, 1817, 167–181. [Google Scholar] [CrossRef]
- Frank, H.A.; Bautista, J.A.; Josue, J.S.; Young, A.J. Mechanism of nonphotochemical quenching in green plants: Energies of the lowest excited singlet states of violaxanthin and zeaxanthin. Biochemistry 2000, 39, 2831–2837. [Google Scholar]
- Holt, N.E.; Zigmantas, D.; Valkunas, L.; Li, X.P.; Niyogi, K.K.; Fleming, G.R. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 2005, 307, 433–436. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Demmig-Adams, B.; Adams, R.B. Eye Nutrition in Context: Mechanisms, Implementation, and Future Directions. Nutrients 2013, 5, 2483-2501. https://doi.org/10.3390/nu5072483
Demmig-Adams B, Adams RB. Eye Nutrition in Context: Mechanisms, Implementation, and Future Directions. Nutrients. 2013; 5(7):2483-2501. https://doi.org/10.3390/nu5072483
Chicago/Turabian StyleDemmig-Adams, Barbara, and Robert B. Adams. 2013. "Eye Nutrition in Context: Mechanisms, Implementation, and Future Directions" Nutrients 5, no. 7: 2483-2501. https://doi.org/10.3390/nu5072483