Effects of Dietary Isoflavones from Puerariae Radix on Lipid and Bone Metabolism in Ovariectomized Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of P. lobata Total Isoflavones (PTIF)
2.2. Animals and Treatments
2.3. BMD Measurements
2.4. Serum Lipid, Estradiol and Bone Marker Analysis
2.5. Liver Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. Compositional Analysis of Total Isoflavones from P. lobata extracts

| Isoflavones | Concentration (%, w/w) |
|---|---|
| Puerarin | 7.2 |
| Daidzin | 3.8 |
| Genistin | 1.5 |
| Total isoflavones | 12.5 |
3.2. Effects of PTIF on Body Weight and Serum Lipid Concentrations in OVX Rats
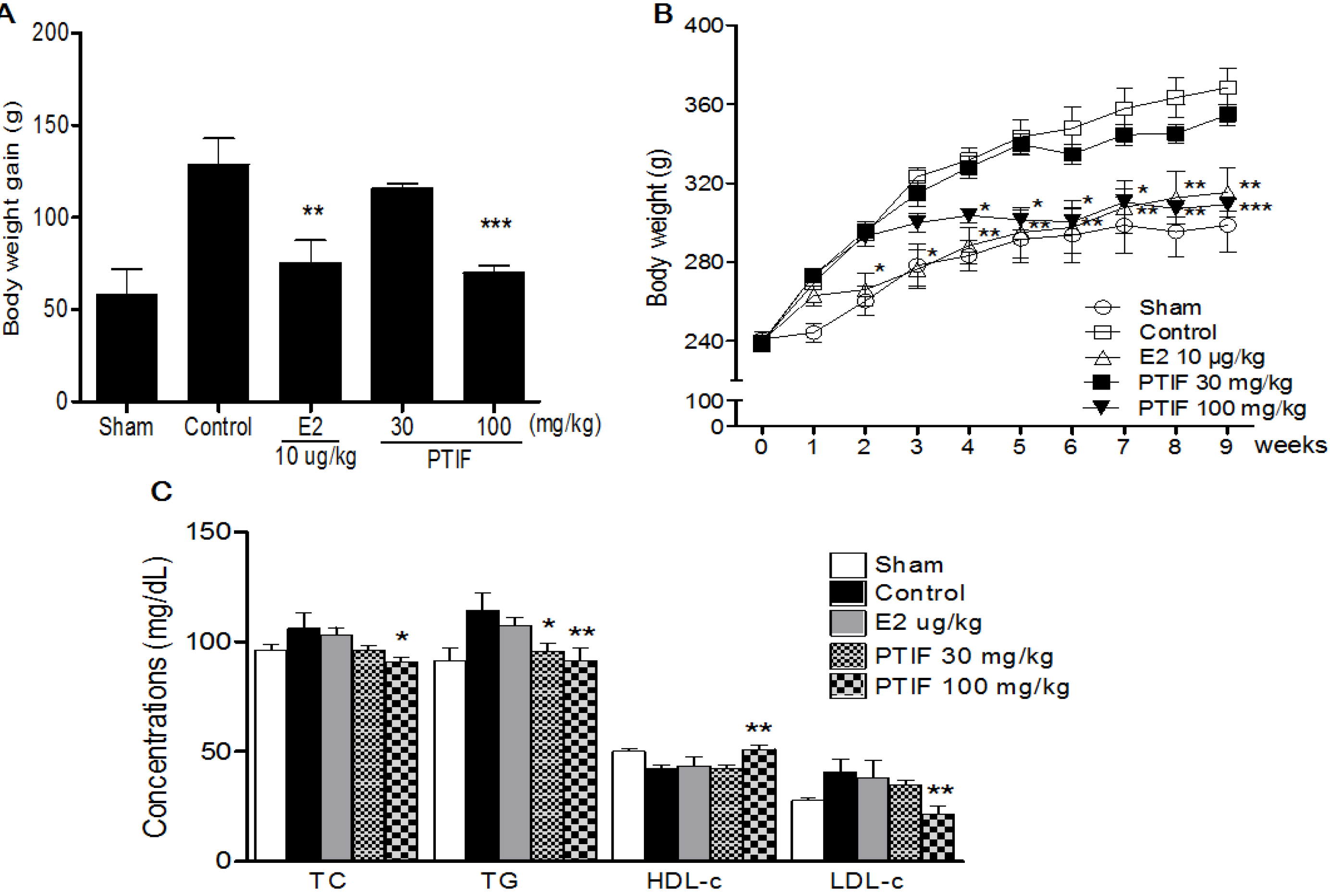
3.3. Effects of PTIF on OVX-Induced Hepatic Steatosis in Rats
| Groups | Serum (mg/dL) | Liver lipid (mg/g wet wt) | ||
|---|---|---|---|---|
| ALT | AST | TC | TG | |
| 109.1 ± 12.5 | 72.1 ± 6.9 | 7.1 ± 0.7 | 35.2 ± 4.9 | |
| Control | 135.5 ± 15.7 | 94.5 ± 6.6 | 11.1 ± 0.8 | 68.8 ± 7.4 |
| E2 10 μg/kg | 121.1 ± 17.4 a | 80.3 ± 4.9 a | 8.2 ± 0.6 a | 51.8 ± 6.8 a |
| PTIF 30 mg/kg | 120.6 ± 19.5 a | 77.5 ± 6.8 a | 7.5 ± 0.5 a | 48.1 ± 5.8 a |
| PTIF 100 mg/kg | 118.4 ± 13.5 b | 76.5 ± 6.8 b | 6.5 ± 0.5 b | 43.1 ± 5.8 b |

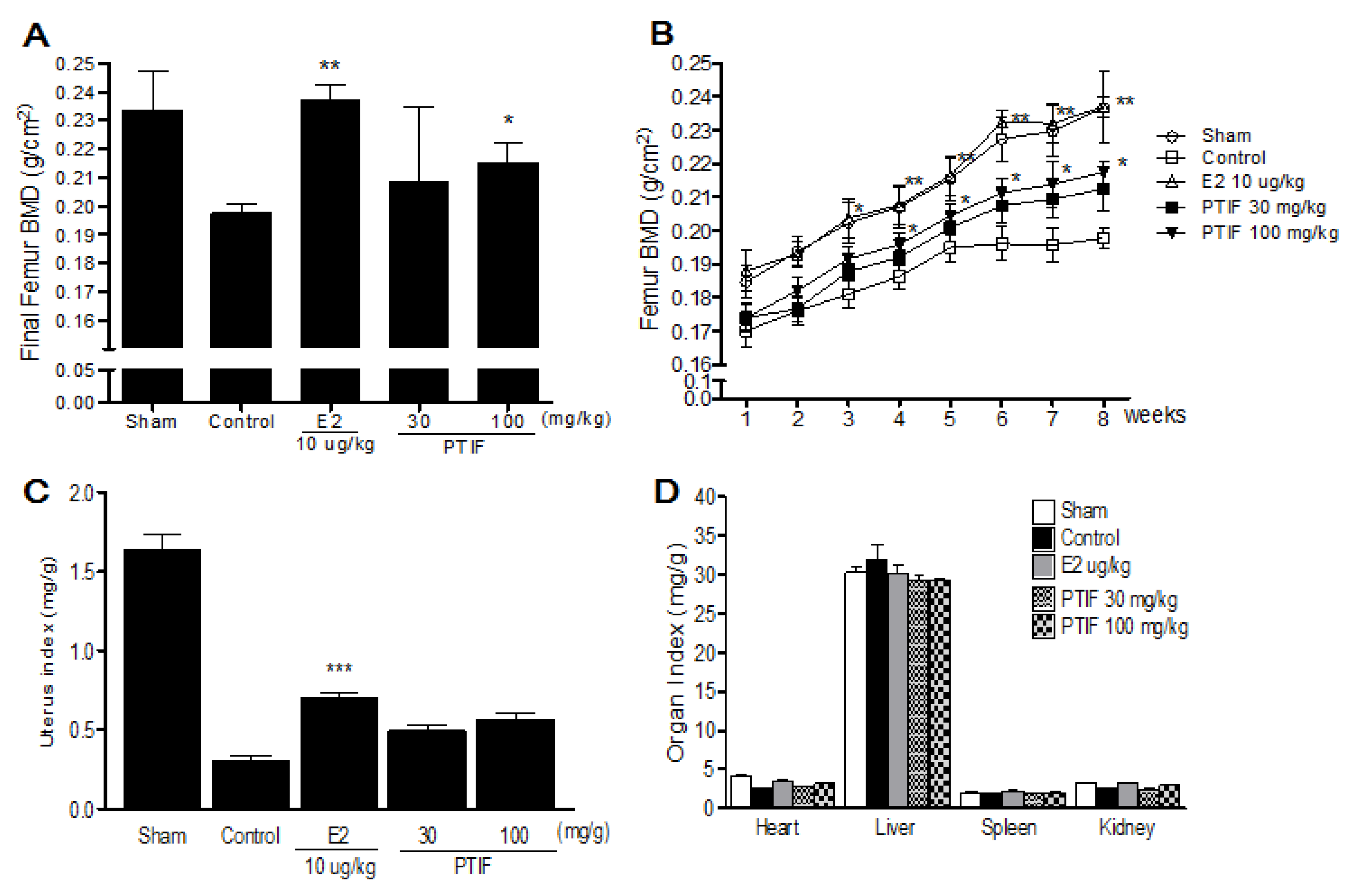
3.4. Effects of PTIF on BMD and Organ Weights in OVX Rats
3.5. Effects of PTIF on Bone Marker in OVX Rats
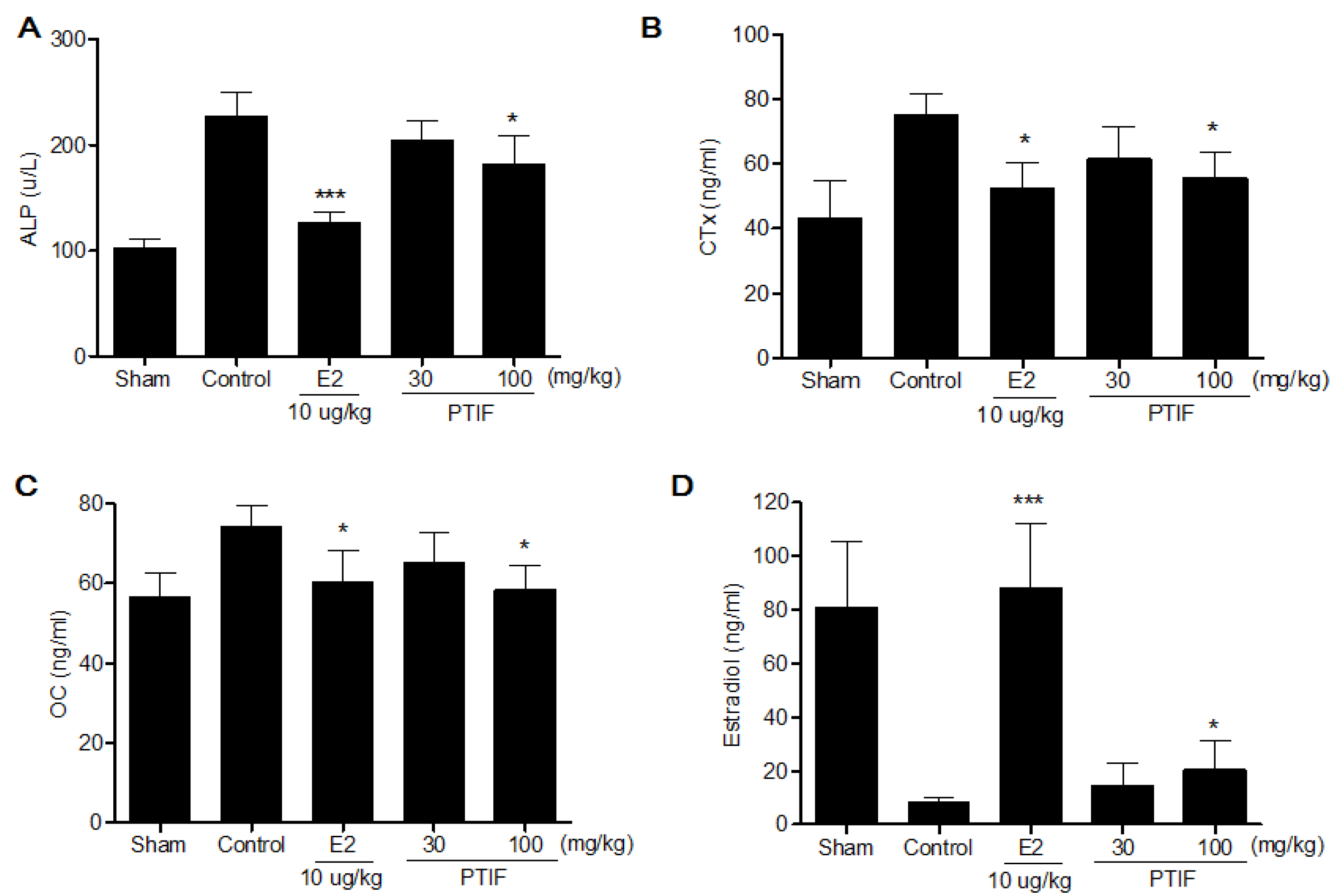
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Pinkerton, J.V.; Guico-Pabia, C.J.; Taylor, H.S. Menstrual cycle-related exacerbation of disease. Am. J. Obstet. Gynecol. 2010, 202, 221–231. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sowers, M. The menopause transition. Endocrinol. Metab. Clin. N. Am. 1997, 26, 261–277. [Google Scholar] [CrossRef]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, S.M.; Allan, J.D. Postmenopausal hormone replacement therapy: Scientific review. JAMA 2002, 288, 872–881. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rossouw, J.E.; Siscovick, D.S.; Mouton, C.P.; Rifai, N.; Wallace, R.B.; Jackson, R.D.; Pettinger, M.B.; Ridker, P.M. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the Women’s Health Initiative observational study. JAMA 2002, 288, 980–987. [Google Scholar]
- Martin, L.J.; Minkin, S.; Boyd, N.F. Hormone therapy, mammographic density, and breast cancer risk. Maturitas 2009, 64, 20–26. [Google Scholar] [CrossRef]
- Hedelin, M.; Klint, A.; Chang, E.T.; Bellocco, R.; Johansson, J.E.; Andersson, S.O.; Heinonen, S.M.; Adlercreutz, H.; Adami, H.O.; Gronberg, H.; et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: The cancer prostate Sweden study (Sweden). Cancer Causes Control 2006, 17, 169–180. [Google Scholar] [CrossRef]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: Meta-analysis of randomized controlled trials. Clin. Nutr. 2008, 27, 57–64. [Google Scholar] [CrossRef]
- Shedd-Wise, K.M.; Alekel, D.L.; Hofmann, H.; Hanson, K.B.; Schiferl, D.J.; Hanson, L.N.; van Loan, M.D. The soy isoflavones for reducing bone loss study: 3-Yr effects on pQCT bone mineral density and strength measures in postmenopausal women. J. Clin. Densitom. 2011, 14, 47–57. [Google Scholar] [CrossRef]
- Bahr, J.M.; Nakai, M.; Rivera, A.; Walsh, J.; Evans, G.L.; Lotinun, S.; Turner, R.T.; Black, M.; Jeffery, E.H. Dietary soy protein and isoflavones: Minimal beneficial effects on bone and no effect on the reproductive tract of sexually mature ovariectomized Sprague-Dawley rats. Menopause 2005, 12, 165–173. [Google Scholar] [CrossRef]
- Alekel, D.L.; van Loan, M.D.; Koehler, K.J.; Hanson, L.N.; Stewart, J.W.; Hanson, K.B.; Kurzer, M.S.; Peterson, C.T. The soy isoflavones for reducing bone loss (SIRBL) study: A 3-y randomized controlled trial in postmenopausal women. Am. J. Clin. Nutr. 2010, 91, 218–230. [Google Scholar] [CrossRef]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Inter. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Vatanparast, H.; Pierson, R.; Case, A.; Olatunbosun, O.; Whiting, S.J.; Beck, T.J.; Pahwa, P.; Biem, H.J. Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: A randomized clinical trial. J. Bone Miner. Res. 2013, 28, 780–793. [Google Scholar] [CrossRef]
- Guerra, M.C.; Speroni, E.; Broccoli, M.; Cangini, M.; Pasini, P.; Minghett, A.; Crespi-Perellino, N.; Mirasoli, M.; Cantelli-Forti, G.; Paolini, M. Comparison between chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sci. 2000, 67, 2997–3006. [Google Scholar] [CrossRef]
- Chung, M.J.; Sung, N.J.; Park, C.S.; Kweon, D.K.; Mantovani, A.; Moon, T.W.; Lee, S.J.; Park, K.H. Antioxidative and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cells and in C57 BL/6J mice. Eur. J. Pharmacol. 2008, 578, 159–170. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, G.; Zhao, H. Effects of Puerariae isoflavone on blood viscosity, thrombosis and platelet function. (in Chinese). Zhong Yao Cai 1997, 20, 468–469. [Google Scholar]
- Yan, L.P.; Chan, S.W.; Chan, A.S.; Chen, S.L.; Ma, X.J.; Xu, H.X. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006, 79, 324–330. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef]
- Jee, W.S.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal Interact. 2001, 1, 193–207. [Google Scholar]
- Thiede, M.A.; Smock, S.L.; Petersen, D.N.; Grasser, W.A.; Thompson, D.D.; Nishimoto, S.K. Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology 1994, 135, 929–937. [Google Scholar] [CrossRef]
- Coleman, R.E. The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 2002, 94, 2521–2533. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Hoegh-Andersen, P.; Tanko, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.M.; Delaisse, J.M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthrit. Res. Ther. 2004, 6, R169–R180. [Google Scholar] [CrossRef] [Green Version]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Nishizawa, Y.; Nakamura, T.; Ohta, H.; Kushida, K.; Gorai, I.; Shiraki, M.; Fukunaga, M.; Hosoi, T.; Miki, T.; Chaki, O.; et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J. Bone Miner. Metab. 2005, 23, 97–104. [Google Scholar]
- Yogesh, H.S.; Chandrashekhar, V.M.; Katti, H.R.; Ganapaty, S.; Raghavendra, H.L.; Gowda, G.K.; Goplakhrishna, B. Anti-osteoporotic activity of aqueous-methanol extract of Berberis aristata in ovariectomized rats. J. Ethnopharmacol. 2011, 134, 334–338. [Google Scholar] [CrossRef]
- Lim, D.W.; Kim, Y.T. Dried root of Rehmannia glutinosa prevents bone loss in ovariectomized rats. Molecules 2013, 18, 5804–5813. [Google Scholar] [CrossRef]
- Hertrampf, T.; Schleipen, B.; Offermanns, C.; Velders, M.; Laudenbach, U.; Diel, P. Comparison of the bone protective effects of an isoflavone-rich diet with dietary and subcutaneous administrations of genistein in ovariectomized rats. Toxicol. Lett. 2009, 184, 198–203. [Google Scholar] [CrossRef]
- Devareddy, L.; Khalil, D.A.; Smith, B.J.; Lucas, E.A.; Soung, D.Y.; Marlow, D.D.; Arjmandi, B.H. Soy moderately improves microstructural properties without affecting bone mass in an ovariectomized rat model of osteoporosis. Bone 2006, 38, 686–693. [Google Scholar] [CrossRef]
- Dang, Z.C.; van Bezooijen, R.L.; Karperien, M.; Papapoulos, S.E.; Lowik, C.W.G.M. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J. Bone Miner. Res. 2002, 17, 394–405. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef]
- Joyner, J.M.; Hutley, L.J.; Cameron, D.P. Estrogen receptors in human preadipocytes. Endocrine 2001, 15, 225–230. [Google Scholar] [CrossRef]
- Nagata, K.; Yamazoe, Y. Pharmacogenetics of sulfotransferase. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 159–176. [Google Scholar] [CrossRef]
- Pasqualini, J.R. Estrogen sulfotransferases in breast and endometrial cancers. Ann. N. Y. Acad. Sci. 2009, 1155, 88–98. [Google Scholar] [CrossRef]
- Lee, S.A.; Choi, J.Y.; Shin, C.S.; Hong, Y.C.; Chung, H.; Kang, D. SULT1E1 genetic polymorphisms modified the association between phytoestrogen consumption and bone mineral density in healthy Korean women. Calcif. Tissue Int. 2006, 79, 152–159. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Getlinger, M.J.; Goyal, N.V.; Alekel, L.; Hasler, C.M.; Juma, S.; Drum, M.L.; Hollis, B.W.; Kukreja, S.C. Role of soy protein with normal or reduced isoflavone content in reversing bone loss induced by ovarian hormone deficiency in rats. Am. J. Clin. Nutr. 1998, 68, 1358S–1363S. [Google Scholar]
- Lee, Y.H.; Hyun, S.H.; Choung, S.Y. Effect of herbal extract mixture on menopausal urinary incontinence in ovariectomized rats. Biofactors 2006, 26, 171–178. [Google Scholar] [CrossRef]
- Hassan, H.A.; El Wakf, A.M.; El Gharib, N.E. Role of phytoestrogenic oils in alleviating osteoporosis associated with ovariectomy in rats. Cytotechnology 2012. [Google Scholar] [CrossRef]
- Li, B.; Yu, S. Genistein prevents bone resorption diseases by inhibiting bone resorption and stimulating bone formation. Biol. Pharm. Bull. 2003, 26, 780–786. [Google Scholar] [CrossRef]
- Wang, J.F.; Guo, Y.X.; Niu, J.Z.; Liu, J.; Wang, L.Q.; Li, P.H. Effects of Radix Puerariae flavones on liver lipid metabolism in ovariectomized rats. World J. Gastroenterol. 2004, 10, 1967–1970. [Google Scholar]
- Radi, Z.A.; Koza-Taylor, P.H.; Bell, R.R.; Obert, L.A.; Runnels, H.A.; Beebe, J.S.; Lawton, M.P.; Sadis, S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am. J. Pathol. 2011, 179, 240–247. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, D.W.; Kim, J.G.; Kim, Y.T. Effects of Dietary Isoflavones from Puerariae Radix on Lipid and Bone Metabolism in Ovariectomized Rats. Nutrients 2013, 5, 2734-2746. https://doi.org/10.3390/nu5072734
Lim DW, Kim JG, Kim YT. Effects of Dietary Isoflavones from Puerariae Radix on Lipid and Bone Metabolism in Ovariectomized Rats. Nutrients. 2013; 5(7):2734-2746. https://doi.org/10.3390/nu5072734
Chicago/Turabian StyleLim, Dong Wook, Jae Goo Kim, and Yun Tai Kim. 2013. "Effects of Dietary Isoflavones from Puerariae Radix on Lipid and Bone Metabolism in Ovariectomized Rats" Nutrients 5, no. 7: 2734-2746. https://doi.org/10.3390/nu5072734





