2013 Update on Celiac Disease and Eosinophilic Esophagitis
Abstract
:1. Introduction
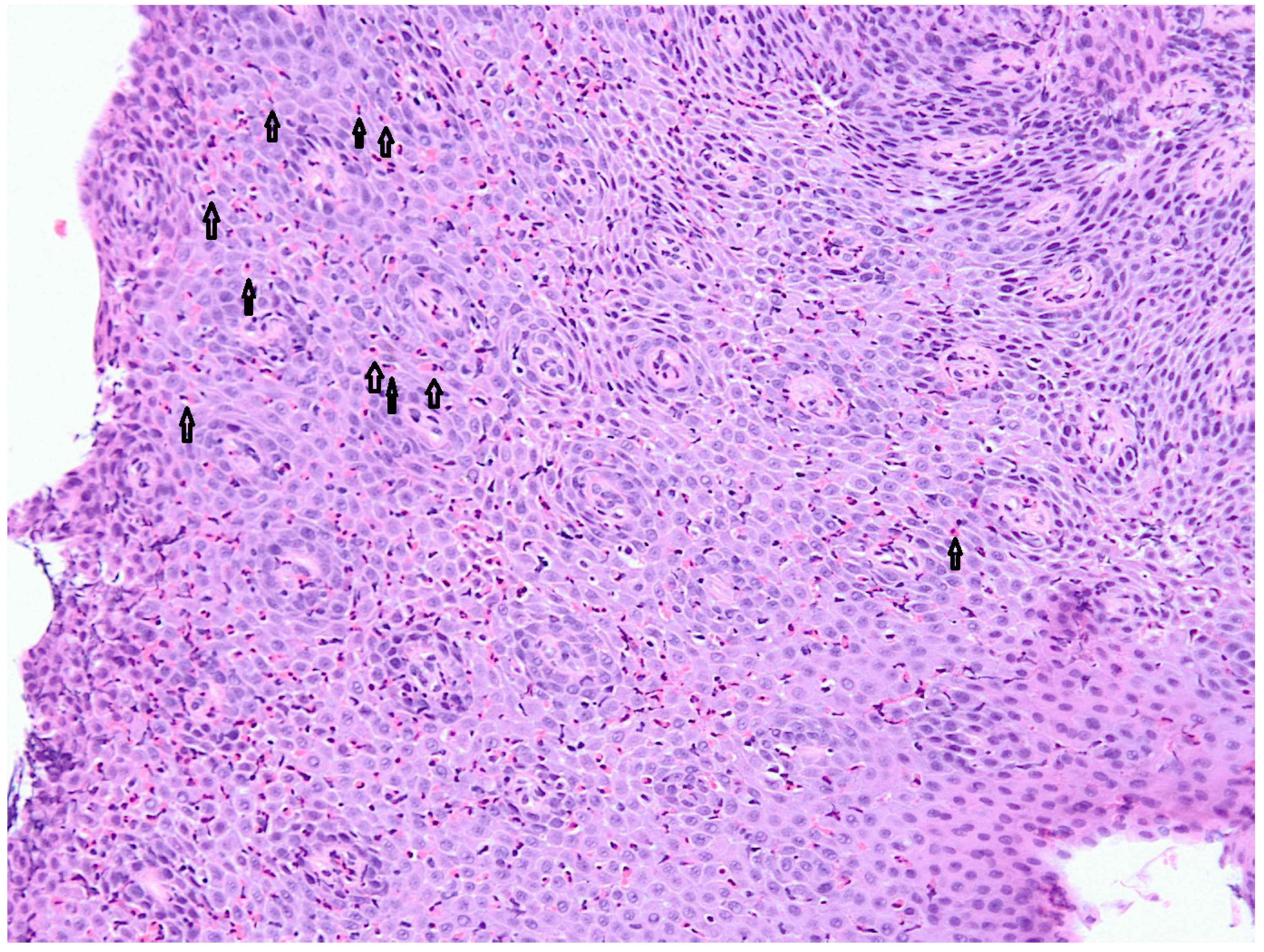
2. Experimental Section
3. Results
| Study | Country | Prevalence of EoE in CD population | % of Pediatric patients | % of Male in EoE patients | Population |
|---|---|---|---|---|---|
| A [22] | Australia | 3.1% (7 of 221) | 100 | 43 | Tertiary center |
| B [23] | Australia | 8.2% (10 of 121) | 100 | 60 | Tertiary center |
| C [25] | USA | 0.97% (14 of 1439) | 20.6 | 57 | Tertiary center |
| D [27] | Canada | 1.2% (3 of 245) | 100 | 100 | General population |
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Fasano, A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005, 128, 68–73. [Google Scholar] [CrossRef]
- Farrell, R.J.; Kelly, C.P. Celiac sprue. N. Engl. J. Med. 2002, 346, 180–188. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Astegiano, M.; Fagoonee, S.; Rizzetto, M.; Pellicano, R. Epilepsy and celiac disease. Panminerva Med. 2011, 53, 213–216. [Google Scholar]
- Landres, R.T.; Kuster, G.G.; Strum, W.B. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978, 74, 1298–1301. [Google Scholar]
- Kelly, K.J.; Lazenby, A.J.; Rowe, P.C.; Yardley, J.H.; Perman, J.A.; Sampson, H.A. Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with amino acid-based formula. Gastroenterology 1995, 109, 1503–1512. [Google Scholar] [CrossRef]
- Furuta, G.T.; Liacouras, C.A.; Collins, M.H.; Gupta, S.K.; Justinich, C.; Putnam, P.E.; Bonis, P.; Hassall, E.; Straumann, A.; Rothenberg, M.E.; et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007, 133, 1342–1363. [Google Scholar] [CrossRef]
- Nurko, S.; Teitelbaum, J.E.; Husain, K.; Buonomo, C.; Fox, V.L.; Antonioli, D.; Fortunato, C.; Badizadegan, K.; Furuta, G.T. Association of Schatzki ring with eosinophilic esophagitis in children. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 436–441. [Google Scholar] [CrossRef]
- Feldman, M.; Friedman, L.S.; Brandt, L.J. Gastrointestinal and Liver Disease, 9th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010; p. 427. [Google Scholar]
- Potter, J.W.; Saeian, K.; Staff, D.; Massey, B.T.; Komorowski, R.A.; Shaker, R.; Hogan, W.J. Eosinophilic esophagitis in adults: An emerging problem with unique esophageal features. Gastrointest.Endosc. 2004, 59, 355–361. [Google Scholar] [CrossRef]
- Orenstein, S.R.; Shalaby, T.M.; Di Lorenzo, C.; Putnam, P.E.; Sigurdsson, L.; Mousa, H.; Kocoshis, S.A. The spectrum of pediatric eosinophilic esophagitis beyond infancy: A clinical series of 30 children. Am. J. Gastroenterol. 2000, 95, 1422–1430. [Google Scholar] [CrossRef]
- Desai, T.K.; Stecevic, V.; Chang, C.H.; Goldstein, N.S.; Badizadegan, K.; Furuta, G.T. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest. Endosc. 2005, 61, 795–801. [Google Scholar] [CrossRef]
- Ronkainen, J.; Talley, N.J.; Aro, P.; Storskrubb, T.; Johansson, S.E.; Lind, T.; Bolling-Sternevald, E.; Vieth, M.; Stolte, M.; Walker, M.M.; et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: The population-based Kalixanda study. Gut 2007, 56, 615–620. [Google Scholar] [CrossRef]
- Liacouras, C.A.; Furuta, G.T.; Hirano, I.; Atkins, D.; Attwood, S.E.; Bonis, P.A.; Burks, A.W.; Chehade, M.; Collins, M.H.; Dellon, E.S.; et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J. Allergy Clin. Immunol. 2011, 128, 3–20. [Google Scholar] [CrossRef]
- Hruz, P.; Straumann, A.; Bussmann, C.; Heer, P.; Simon, H.U.; Zwahlen, M.; Beglinger, C.; Schoepfer, A.M. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J. Allergy Clin. Immunol. 2011, 128, 1349–1350. [Google Scholar] [CrossRef]
- Noel, R.J.; Putnam, P.E.; Rothenberg, M.E. Eosinophilic esophagitis. N. Engl. J. Med. 2004, 351, 940–941. [Google Scholar] [CrossRef]
- Cherian, S.; Smith, N.M.; Forbes, D.A. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch. Dis. Child. 2006, 91, 1000–1004. [Google Scholar] [CrossRef]
- Prasad, G.A.; Alexander, J.A.; Schleck, C.D.; Zinsmeister, A.R.; Smyrk, T.C.; Elias, R.M.; Locke, G.R.; Talley, N.J. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin. Gastroenterol. Hepatol. 2009, 7, 1055–1061. [Google Scholar] [CrossRef]
- Straumann, A.; Simon, H.U. Eosinophilic esophagitis: Escalating epidemiology? J. Allergy Clin. Immunol. 2005, 115, 418–419. [Google Scholar] [CrossRef]
- Kagalwalla, A.F.; Shah, A.; Ritz, S.; Melin-Aldana, H.; Li, B.U. Cow’s milk protein-induced eosinophilic esophagitis in a child with gluten-sensitive enteropathy. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 386–388. [Google Scholar] [CrossRef]
- De Angelis, P.; Dall’Oglio, L.; Di Leo, G.; Ventura, A. Eosinophilic oesophagitis and coeliac disease: Is it just a casual association? Gut 2007, 56, 1029–1030. [Google Scholar] [CrossRef]
- Verzegnassi, F.; Bua, J.; Tommasini, A.; Not, T.; Kiren, V.; Baldas, V.; Santon, D.; Trevisiol, C.; Berti, I.; Neri, E.; et al. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch. Dis. Child. 2004, 89, 512–515. [Google Scholar]
- Quaglietta, L.; Coccorullo, P.; Miele, E.; Pascarella, F.; Troncone, R.; Staiano, A. Eosinophilic oesophagitis and coeliac disease: Is there an association? Aliment. Pharmacol. Ther. 2007, 26, 487–493. [Google Scholar]
- Ooi, C.Y.; Day, A.S.; Jackson, R.; Bohane, T.D.; Tobias, V.; Lemberg, D.A. Eosinophilic esophagitis in children with celiac disease. J. Gastroenterol. Hepatol. 2008, 23, 1144–1148. [Google Scholar] [CrossRef]
- Leslie, C.; Mews, C.; Charles, A.; Ravikumara, M. Celiac disease and eosinophilic esophagitis: A true association. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 397–399. [Google Scholar]
- Lucendo, A.J.; Arias, Á.; Pérez-Martínez, I.; López-Vázquez, A.; Ontañón-Rodríguez, J.; González-Castillo, S.; De Rezende, L.C.; Rodrigo, L. Adult patients with eosinophilic esophagitis do not show an increased frequency of the HLA-DQ2/DQ8 genotypes predisposing to celiac disease. Dig. Dis. Sci. 2011, 56, 1107–1111. [Google Scholar] [CrossRef]
- Thompson, J.S.; Lebwohl, B.; Reilly, N.R.; Talley, N.J.; Bhagat, G.; Green, P.H. Increased incidence of eosinophilic esophagitis in children and adults with celiac disease. J. Clin. Gastroenterol. 2012, 46, 6–11. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Aro, P.; Walker, M.M.; Vieth, M.; Agréus, L.; Talley, N.J.; Murray, J.A.; Ronkainen, J. Celiac disease, eosinophilic esophagitis and gastroesophageal reflux disease, an adult population-based study. Scand. J. Gastroenterol. 2013, 48, 804–814. [Google Scholar]
- Stewart, M.J.; Shaffer, E.; Urbanski, S.J.; Beck, P.L.; Storr, M.A. The association between celiac disease and eosinophilic esophagitis in children and adults. BMC Gastroenterol. 2013, 13, 96. [Google Scholar] [CrossRef]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Eosinophilic gastrointestinal disorders (EGID). J. Allergy Clin. Immunol. 2004, 113, 11–28. [Google Scholar] [CrossRef]
- Spergel, J.M.; Beausoleil, J.L.; Mascarenhas, M.; Liacouras, C.A. The use of skin prick tests and patch tests to identify the causative foods in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2002, 109, 363–368. [Google Scholar] [CrossRef]
- Van Elburg, R.M.; Uil, J.J.; Mulder, C.J.; Heymans, H.S. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 1993, 34, 354–357. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Mayer, J.; Nguyen, Q.T.; Stolte, M.; Manns, M.P. Immunohistological assessment of intestinal eosinophil activation in patients with eosinophilic gastroenteritis and inflammatory bowel disease. Am. J. Gastroenterol. 1999, 94, 3521–3529. [Google Scholar] [CrossRef]
- Heine, R.G. Eosinophilic esophagitis in children with celiac disease: New diagnostic and therapeutic dilemmas. J. Gastroenterol. Hepatol. 2008, 23, 993–994. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pellicano, R.; De Angelis, C.; Ribaldone, D.G.; Fagoonee, S.; Astegiano, M. 2013 Update on Celiac Disease and Eosinophilic Esophagitis. Nutrients 2013, 5, 3329-3336. https://doi.org/10.3390/nu5093329
Pellicano R, De Angelis C, Ribaldone DG, Fagoonee S, Astegiano M. 2013 Update on Celiac Disease and Eosinophilic Esophagitis. Nutrients. 2013; 5(9):3329-3336. https://doi.org/10.3390/nu5093329
Chicago/Turabian StylePellicano, Rinaldo, Claudio De Angelis, Davide Giuseppe Ribaldone, Sharmila Fagoonee, and Marco Astegiano. 2013. "2013 Update on Celiac Disease and Eosinophilic Esophagitis" Nutrients 5, no. 9: 3329-3336. https://doi.org/10.3390/nu5093329








