Effects of Dietary Zinc Manipulation on Growth Performance, Zinc Status and Immune Response during Giardia lamblia Infection: A Study in CD-1 Mice
Abstract
:1. Introduction
2. Experimental Section
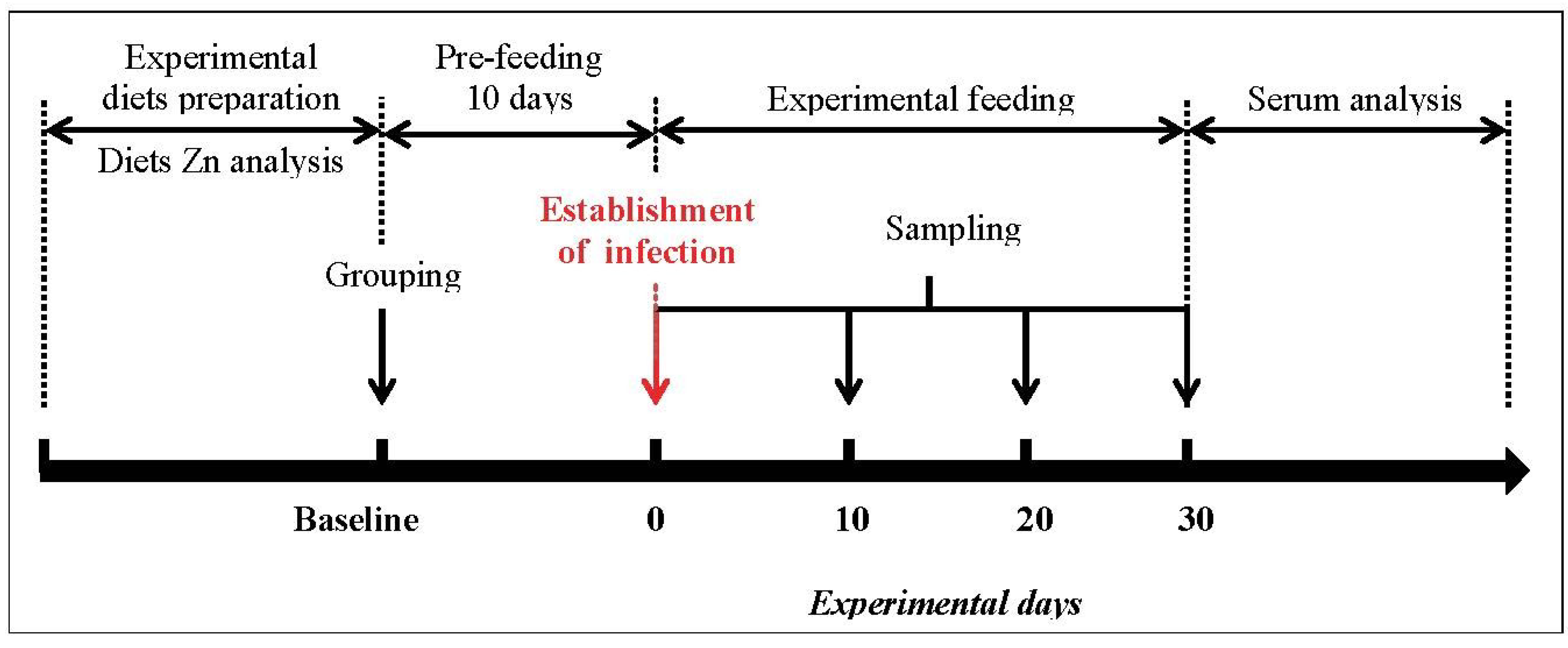
2.1. Mice, Diets and Study Design
| Ingredient | g/kg diet |
|---|---|
| Corn starch | 653 |
| Casein | 200 |
| Corn oil | 50 |
| Cellulose | 50 |
| Vitamin mix 1 | 10 |
| Mineral mix 2 | 35 |
| Zinc gluconate | * |
| Choline bitartrate | 2 |
2.2. Establishment of Giardia lamblia Infection
2.3. Diet and Serum Analysis for Zinc Content
2.4. Immunoglobulin ELISA
2.5. Statistical Analysis
3. Results
3.1. Zinc Content of Experimental Diets
3.2. Growth Performance as Affected by Dietary Zinc and Infection
| Diet | Giardia-free | Giardia-infected | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Initial BW | Final BW | Gain | n | Initial BW | Final BW | Gain | ||||
| ZnL | 9 | 34.9 ± 2.1 | 38.5 ± 1.9 b | 3.6 | <0.001 | 10 | 35.0 ± 2.0 | 33.9 ± 1.6 a | −1.2 | 0.214 | |
| ZnA | 10 | 35.8 ± 3.0 | 39.2 ± 2.8 b | 3.4 | <0.001 | 10 | 34.2 ± 2.6 | 34.5 ± 2.9 a | 0.3 | 0.182 | |
| ZnH | 10 | 32.5 ± 1.5 | 43.6 ± 2.6 c | 11.1 | <0.001 | 10 | 33.0 ± 2.1 | 42.8 ± 2.6 c | 9.6 | <0.001 | |
| ZnS | 8 | 33.3 ± 1.9 | 43.1 ± 2.9 c | 9.8 | 0.008 | 8 | 34.0 ± 1.7 | 43.1 ± 2.2 c | 9.1 | 0.002 | |
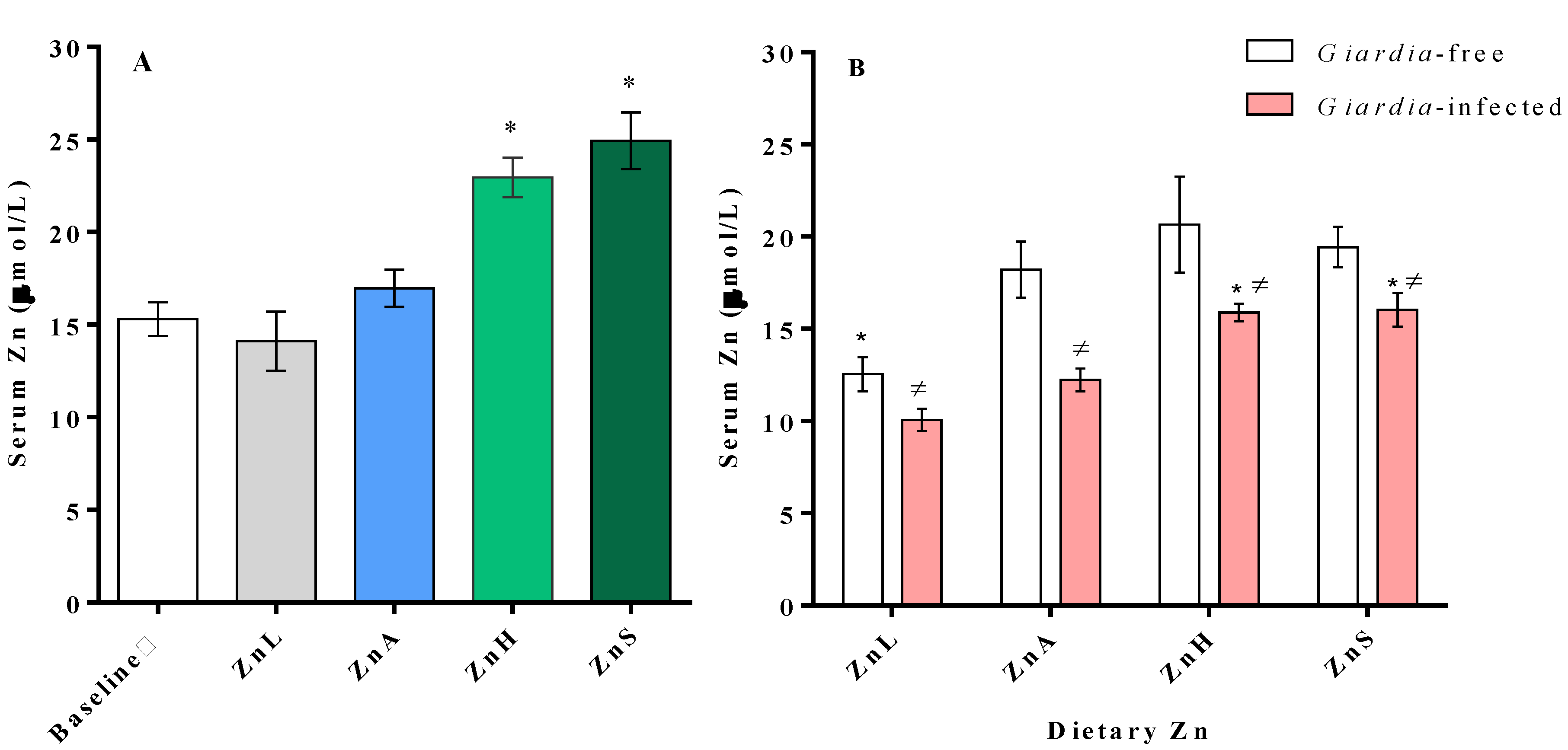
3.3. Serum Zinc Changes Associated with Dietary Zinc Level and Infection
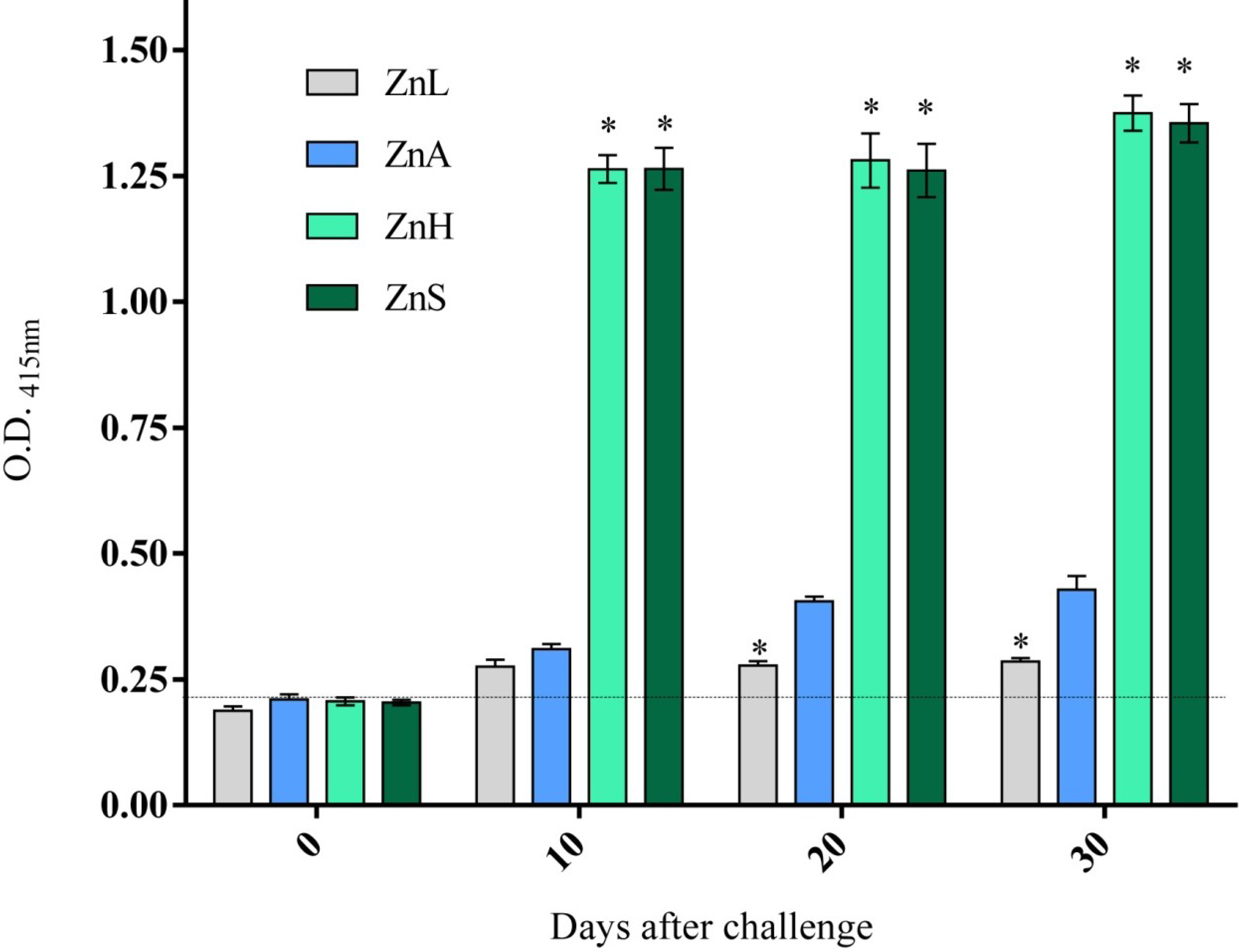
3.4. Immune Response as Affected by Dietary Zinc
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002, 56, 73–76. [Google Scholar] [CrossRef]
- Hugues, S.; Kelly, P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 2006, 28, 577–588. [Google Scholar]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452–1456. [Google Scholar]
- Solomons, N.W. Update on zinc biology. Ann. Nutr. Metab. 2013, 62, 8–17. [Google Scholar] [CrossRef]
- Hesham, M.S.; Edariah, A.B.; Norhayati, M. Intestinal parasitic infections and micronutrient deficiency: A review. Med. J. Malaysia 2004, 59, 284–293. [Google Scholar]
- Jendryczko, A.; Sodowska, H.; Drózdz, M. Zinc deficiency in children infected with Giardia lamblia. Wiad. Lek. 1993, 46, 32–35. [Google Scholar]
- Karakas, Z.; Demirel, N.; Tarakcioglu, M.; Mete, N. Serum zinc and copper levels in southeastern Turkish children with giardiasis or amebiasis. Biol. Trace Elem. Res. 2001, 84, 11–18. [Google Scholar] [CrossRef]
- Ertan, P.; Yereli, K.; Kurt, O.; Balcioglu, I.; Onag, A. Serological levels of zinc, copper and iron elements among Giardia lamblia infected children in Turkey. Pediatr. Int. 2002, 44, 286–288. [Google Scholar] [CrossRef]
- Berkman, D.S.; Lescano, A.G.; Gilman, R.H.; López, S.L. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: A follow-up study. Lancet 2003, 359, 564–571. [Google Scholar]
- Demirci, M.; Delibas, N.; Altuntas, I.; Oktem, F.; Yonden, Z. Serum iron, zinc and copper levels and lipid peroxidation in children with chronic giardiasis. J. Health Popul. Nutr. 2003, 21, 72–75. [Google Scholar]
- Abou-Shady, O.; El Raziky, M.S.; Zaki, M.M.; Mohamed, R.K. Impact of Giardia lamblia on growth, serum levels of zinc, copper, and iron in Egyptian children. Biol. Trace Elem. Res. 2011, 140, 1–6. [Google Scholar] [CrossRef]
- Quihui, L.; Morales, G.G.; Méndez, R.O.; Leyva, J.G.; Esparza, J.; Valencia, M.E. Could giardiasis be a risk factor for low zinc status in schoolchildren from northwestern Mexico? A cross-sectional study with longitudinal follow up. BMC Public Health 2010, 10. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and Giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Cotton, A.J.; Beatty, J.K.; Buret, A.G. Host parasite interactions and pathophysiology in Giardia infections. Int. J. Parasitol. 2011, 41, 925–932. [Google Scholar] [CrossRef]
- Selmi, C.; Invernizzi, P.; Zuin, M.; Ansari, A.A.; Gershwin, M.E. Evaluation of the Immune Function in the Nutritionally at-Risk Patien. In Handbook of Nutrition and Immunity, 1st ed.; Gershwin, M.E., Nestel, P., Keen, C.L., Eds.; Humana Press: Totowa, NJ, USA, 2004; Volume 1, p. 11. [Google Scholar]
- Caulfield, L.E.; Black, R.E. Zinc Deficiency. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J., Eds.; World Health Organization: Geneva, Switzerland, 2004; pp. 257–259. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar]
- National Research Council, Nutrient Requirements of Laboratory Animals, 4th ed.; National Academy Press: Washington, DC, USA, 1995.
- Astiazarán, G.H.; Espinosa, C.M.; Castañón, G.; Chávez, M.B.; Martínez, P.A. Giardia lamblia: Effect of infection with symptomatic and asymptomatic isolates on the growth of Gerbils (Meriones unguiculatus). Exp. Parasitol. 2000, 95, 128–135. [Google Scholar] [CrossRef]
- AOAC-968.08. Minerals in Animal Feed. Atomic Absorption Spectrophotometric Method. In Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC International: Arlington, VA, USA, 1990; p. 69.
- D’Haese, P.C.; Lamberts, L.V.; Vanheule, A.O.; de Broe, M.E. Direct determination of zinc in serum by Zeeman atomic absorption spectrometry with a graphite furnace. Clin. Chem. 1992, 38, 2439–2443. [Google Scholar]
- Gottstein, B.; Harriman, G.R.; Conrad, J.T.; Nash, T.E. Antigenic variation in Giardia lamblia: Cellular and humoral immune response in a mouse model. Parasite Immunol. 1990, 12, 659–673. [Google Scholar] [CrossRef]
- Velázquez, C.; Beltran, M.; Ontiveros, N.; Rascón, L.; Figueroa, D.C.; Granados, A.J.; Hernández, M.J.; Hernández, J.; Astiazaran, G.H. Giardia lamblia infection induces different secretory and systemic antibody responses in mice. Parasite Immunol. 2005, 27, 351–356. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency in human subjects. Prog. Clin. Biol. Res. 1983, 129, 1–33. [Google Scholar]
- Reeves, P.G.; Odell, B.L. The effect of zinc deficiency on glucose metabolism in meal-fed rats. Br. J. Nutr. 1983, 49, 441–452. [Google Scholar] [CrossRef]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500–1508. [Google Scholar]
- Barthold, S.W. Giardia muris Infection, Intestine, Mouse, Rat, and Hamster. In Monographs on Pathology of Laboratory Animals: Digestive System; Jones, T.C., Mohr, V., Hunt, R.D., Eds.; Springer-Verlag: New York, NY, USA, 1985; pp. 359–362. [Google Scholar]
- Aloisio, F.; Filippini, G.; Antenucci, P.; Lepri, E.; Pezzotti, G.; Cacciò, S.M.; Pozio, E. Severe weight loss in lambs infected with Giardia duodenalis assemblage B. Vet. Parasitol. 2006, 142, 154–158. [Google Scholar] [CrossRef]
- Buret, A.N.; den Hollander, N.; Wallis, P.M.; Befus, D.; Olson, M.E. Zoonotic potential of giardiasis in domestic ruminants. J. Infect. Dis. 1990, 162, 231–237. [Google Scholar] [CrossRef]
- Buret, A.G.; Cotton, J. Pathophysiological Processes and Clinical Manifestations of Giardiasis. In Giardia. A Model Organism, 1st ed.; Lujan, H.D., Svard, S.G., Eds.; Springer Wien: New York, NY, USA, 2011; pp. 300–310. [Google Scholar]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360–1366. [Google Scholar]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Cousins, R.J. Gastrointestinal factors influencing zinc absorption and homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 244–248. [Google Scholar] [CrossRef]
- Kirchgessner, M. Underwood Memorial Lecture. Homeostasis and Homeorhesis in Trace Element Metabolism. In Trace Elements in Man and Animals TEMA 8, Proceedings of the Eighth International Symposium on Trace Elements in Man and Animals, Dresden, Germany, 16–22 May 1993; Anke, M., Meissner, D., Mills, C.F., Eds.; Verlag Media Touristik: Dresden, Germany, 1993; pp. 4–21. [Google Scholar]
- Quihui, C.L.; Méndez, R.O.; Astiazarán, G.H.; Morales, G.G.; Moreno, M.J.; Cuadras, D.; Canett, R. Changes in serum zinc levels associated with giardiasis and dietary zinc intake in mice. Biol. Trace Elem. Res. 2012, 145, 396–402. [Google Scholar] [CrossRef]
- Langford, T.D.; Housley, M.P.; Boes, M.; Chen, J.; Kagnoff, M.F.; Gillin, F.D.; Eckmann, L. Central importance of immunoglobulin A in host defense against Giardia spp. Infect. Immun. 2002, 70, 11–18. [Google Scholar] [CrossRef]
- Heyworth, M.F. Antibody response to Giardia muris trophozoites in mouse intestine. Infect. Immun. 1986, 52, 568–571. [Google Scholar]
- Snider, D.P.; Underdown, B.J. Quantitative and temporal analyses of murine antibody response in serum and gut secretions to infection with Giardia muris. Infect. Immun. 1986, 52, 271–278. [Google Scholar]
- Daniels, C.W.; Belosevic, M. Serum antibody responses by male and female C57BL/6 mice infected with Giardia muris. Clin. Exp. Immunol. 1994, 97, 424–429. [Google Scholar] [CrossRef]
- Faubert, G. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 2000, 13, 35–54. [Google Scholar] [CrossRef]
- Nash, T.E.; Herrington, D.A.; Losonsky, G.A.; Levine, M.M. Experimental human infections with Giardia lamblia. J. Infect. Dis. 1987, 156, 974–984. [Google Scholar] [CrossRef]
- Fraker, P.J.; King, L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004, 24, 277–298. [Google Scholar] [CrossRef]
- King, L.; Frentzel, J.; Mann, J.; Fraker, P. Chronic zinc deficiency in mice disrupted T Cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005, 24, 494–502. [Google Scholar] [CrossRef]
- Faber, C.; Gabriel, P.; Ibs, K.H.; Rink, L. Zinc in pharmacological doses suppresses allogeneic reaction without affecting the antigenic response. Bone Marrow Transpl. 2004, 33, 1241–1246. [Google Scholar] [CrossRef]
- Hodkinson, C.F.; Kelly, M.; Alexander, H.D.; Bradbury, I.; Robson, P.J.; Bonham, M.P.; O’Connor, J.M.; Coudray, C.; Strain, J.J.; Wallace, J.M. Effect of zinc supplementation on the immune status of healthy older individuals aged 55–70 years: The ZENITH Study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 598–608. [Google Scholar] [CrossRef]
- Specialty Feeds. High Zinc Semi-Pure Rodent Diet; Specialty Feeds Pty Ltd.: Glen Forrest, WA, Australia, 2002. Available online: www.specialtyfeeds.com/data/sf00-213.pdf (accessed on 1 April 2013).
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iñigo-Figueroa, G.; Méndez-Estrada, R.O.; Quihui-Cota, L.; Velásquez-Contreras, C.A.; Garibay-Escobar, A.; Canett-Romero, R.; Astiazarán-García, H. Effects of Dietary Zinc Manipulation on Growth Performance, Zinc Status and Immune Response during Giardia lamblia Infection: A Study in CD-1 Mice. Nutrients 2013, 5, 3447-3460. https://doi.org/10.3390/nu5093447
Iñigo-Figueroa G, Méndez-Estrada RO, Quihui-Cota L, Velásquez-Contreras CA, Garibay-Escobar A, Canett-Romero R, Astiazarán-García H. Effects of Dietary Zinc Manipulation on Growth Performance, Zinc Status and Immune Response during Giardia lamblia Infection: A Study in CD-1 Mice. Nutrients. 2013; 5(9):3447-3460. https://doi.org/10.3390/nu5093447
Chicago/Turabian StyleIñigo-Figueroa, Gemma, Rosa O. Méndez-Estrada, Luis Quihui-Cota, Carlos A. Velásquez-Contreras, Adriana Garibay-Escobar, Rafael Canett-Romero, and Humberto Astiazarán-García. 2013. "Effects of Dietary Zinc Manipulation on Growth Performance, Zinc Status and Immune Response during Giardia lamblia Infection: A Study in CD-1 Mice" Nutrients 5, no. 9: 3447-3460. https://doi.org/10.3390/nu5093447





