Association between Serum 25-Hydroxyvitamin D and Inflammatory Cytokines in Healthy Adults
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Body Composition
2.3. Blood Sampling
2.4. Lifestyle Variables (Physical Activity, Vitamin D Intake, Alcohol Consumption, and Smoking Status)
2.5. Statistical Analysis
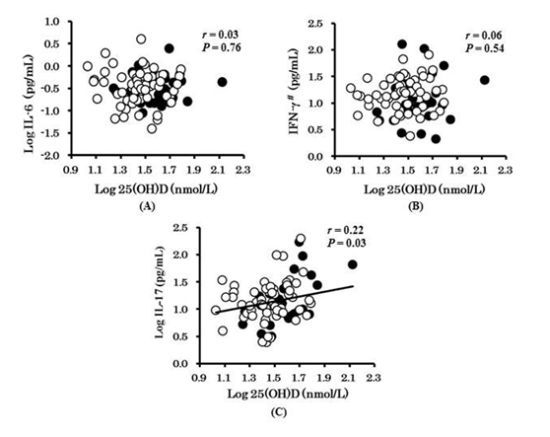
3. Results
| Variable | Total (n = 95) | Men (n = 34) | Women (n = 61) |
|---|---|---|---|
| Age (years) | 44 ± 14 | 42 ± 16 | 44 ± 13 |
| Height (cm) # | 162.5 ± 7.9 | 170.3 ± 5.9 | 158.1 ± 4.9 |
| Weight (kg) # | 58.7 ± 10.4 | 69.0 ± 6.4 | 53.0 ± 7.3 |
| BMI (kg/m2) # | 22.1 ± 3.0 | 23.9 ± 2.6 | 21.2 ± 2.8 |
| Body fat (%) # | 23.4 ± 6.4 | 17.9 ± 4.6 | 26.5 ± 5.0 |
| 25(OH)D (nmol/L) # | 34.7 ± 16.4 | 42.1 ± 20.2 | 30.6 ± 12.1 |
| 1,25(OH)2D (pg/mL) | 38.9 ± 9.8 | 40.8 ± 10.1 | 37.8 ± 9.6 |
| IL-6 (pg/mL) | 0.47 ± 0.53 | 0.42 ± 0.42 | 0.49 ± 0.58 |
| IFN-γ (pg/mL) | 1.14 ± 1.34 | 1.49 ± 1.86 | 0.95 ± 0.89 |
| IL-17 (pg/mL) | 21.7 ± 30.1 | 23.3 ± 32.7 | 20.9 ± 28.8 |
| iPTH (pg/mL) | 63.1 ± 21.6 | 58.1 ± 21.2 | 66.0 ± 21.5 |
| MVPA (min/day) † | 33.7 ± 21.1 | 34.7 ± 23.6 | 33.1 ± 19.8 |
| Vitamin D intake (μg/day) | 6.5 ± 4.2 | 5.3 ± 2.4 | 7.1 ± 4.9 |

| Log IL-17 | B | β | P |
|---|---|---|---|
| Model 1 | |||
| Log serum 25(OH)D (nmol/L) | 0.592 | 0.288 | 0.010 |
| Model 2 | |||
| Log serum 25(OH)D (nmol/L) | 0.543 | 0.264 | 0.025 |
| Model 3 | |||
| Log serum 25(OH)D (nmol/L) | 0.579 | 0.282 | 0.019 |
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.F. Nonclassical vitamin D actions. Nutrients 2010, 2, 408–425. [Google Scholar] [CrossRef]
- Colin, E.M.; Asmawidjaja, P.S.; van Hamburg, J.P.; Mus, A.M.; van Driel, M.; Hazes, J.M.; van Leeuwen, J.P.; Lubberts, E. 1,25-Dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010, 62, 132–142. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.; Gaitan, M. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain 2009, 132, 1146–1160. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.Y.A.; Koenen, H.J.P.M.; Sweep, F.C.G.J.; Joosten, I.; Netea, M.G.; van der Ven, A.J.A.M. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011, 164, 72–79. [Google Scholar] [CrossRef]
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1α,25-dihydroxyvitamin D3 target the TNF-α pathway to suppress experimental inflammatory bowel disease. Eur. J. Immunol. 2004, 35, 217–224. [Google Scholar]
- Lemire, J.M. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J. Cell. Biochem. 1992, 49, 26–31. [Google Scholar] [CrossRef]
- Muller, K.; Diamant, M.; Bendtzen, K. Inhibition of production and function of interleukin-6 by 1,25-dihydroxyvitamin D3. Immunol. Lett. 1991, 28, 115–120. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Dixon, B.M.; Schneider, E.D.; Henriksen, V.T.; Weaver, L.K. Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur. J. Appl. Physiol. 2013, 113, 1523–1534. [Google Scholar] [CrossRef]
- Peterson, C.A.; Heffernan, M.E. Serum tumor necrosis factor-α concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J. Inflamm. 2008, 5, 10. [Google Scholar] [CrossRef]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Kavouras, S.; Stefanadis, C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: The attica study. Prev. Med. 2005, 40, 432–437. [Google Scholar] [CrossRef]
- Golzari, Z.; Shabkhiz, F.; Soudi, S.; Kordi, M.R.; Hashemi, S.M. Combined exercise training reduces IFN-γ and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int. Immunopharmacol. 2010, 10, 1415–1419. [Google Scholar] [CrossRef]
- Scragg, R.; Camargo, C.A., Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the us population: Results from the third national health and nutrition examination survey. Am. J. Epidemiol. 2008, 168, 577–586; discussion 587–591. [Google Scholar] [CrossRef]
- Kumahara, H.; Schutz, Y.; Ayabe, M.; Yoshioka, M.; Yoshitake, Y.; Shindo, M.; Ishii, K.; Tanaka, H. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: A validation study against whole-body indirect calorimetry. Br. J. Nutr. 2004, 91, 235–243. [Google Scholar] [CrossRef]
- Cao, Z.B.; Miyatake, N.; Higuchi, M.; Miyachi, M.; Ishikawa-Takata, K.; Tabata, I. Predicting VO2max with an objectively measured physical activity in Japanese women. Med. Sci. Sports Exerc. 2010, 42, 179–186. [Google Scholar]
- Murakami, K.; Mizoue, T.; Sasaki, S.; Ohta, M.; Sato, M.; Matsushita, Y.; Mishima, N. Dietary intake of folate, other B vitamins, and ω-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition 2008, 24, 140–147. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 days dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Nakanishi, N.; Suzuki, K.; Tatara, K. Alcohol consumption and risk of development of impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. Diabetes Care 2003, 26, 48–54. [Google Scholar] [CrossRef]
- Nakamura, K. Vitamin D insufficiency in Japanese populations: From the viewpoint of the prevention of osteoporosis. J. Bone Miner. Metab. 2006, 24, 1–6. [Google Scholar] [CrossRef]
- Lips, P.; Hosking, D.; Lippuner, K.; Norquist, J.M.; Wehren, L.; Maalouf, G.; Ragi-Eis, S.; Chandler, J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J. Int. Med. 2006, 260, 245–254. [Google Scholar] [CrossRef]
- Nakamura, K.; Tsugawa, N.; Saito, T.; Ishikawa, M.; Tsuchiya, Y.; Maruyama, K.; Oshiki, R.; Kobayashi, R.; Nashimoto, M.; Yoshihara, A.; et al. Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi study. Bone 2008, 42, 271–277. [Google Scholar] [CrossRef]
- Nakamura, K.; Nashimoto, M.; Hori, Y.; Muto, K.; Yamamoto, M. Serum 25-hydroxyvitamin D levels in active women of middle and advanced age in a rural community in Japan. Nutrition 1999, 15, 870–873. [Google Scholar] [CrossRef]
- Nakamura, K.; Nashimoto, M.; Matsuyama, S.; Yamamoto, M. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: A cross sectional study. Nutrition 2001, 17, 921–925. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and health: Evolution, biologic functions, and recommended dietary intakes for vitamin D. Clin. Rev. Bone Miner. Metab. 2009, 7, 2–19. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar]
- Japanese Ministry of Health, Labour and Welfare. Dietary Reference Intakes for Japanese, 2010; Daiichi Shuppan Publishing Co. Ltd.: Tokyo, Japan, 2009.
- Maalmi, H.; Berraies, A.; Tangour, E.; Ammar, J.; Abid, H.; Hamzaoui, K.; Hamzaoui, A. The impact of vitamin D deficiency on immune T cells in asthmatic children: A case-control study. J. Asthma Allergy 2012, 5, 11–19. [Google Scholar]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.Y.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Bhan, I.; Camargo, C.A., Jr.; Wenger, J.; Ricciardi, C.; Ye, J.; Borregaard, N.; Thadhani, R. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J. Allergy Clin. Immunol. 2011, 127, 1302–1304. [Google Scholar]
- Dixon, B.M.; Barker, T.; Mckinnon, T.; Cuomo, J.; Frei, B.; Borregaard, N.; Gombart, A.F. Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP/LL-37) and 25-hydroxyvitamin D levels in healthy adults. BMC Res. Notes 2012, 5, 575. [Google Scholar] [CrossRef]
- Peric, M.; Koglin, S.; Kim, S.M.; Morizane, S.; Besch, R.; Prinz, J.C.; Ruzicka, T.; Gallo, R.L.; Schauber, J. IL-17a enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 2008, 181, 8504–8512. [Google Scholar]
- Robinson-Cohen, C.; Hoofnagle, A.N.; Ix, J.H.; Sachs, M.C.; Tracy, R.P.; Siscovick, D.S.; Kestenbaum, B.R.; de Boer, I.H. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA 2013, 310, 179–188. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, X.; Cao, Z.-B.; Zhang, Y.; Ishimi, Y.; Tabata, I.; Higuchi, M. Association between Serum 25-Hydroxyvitamin D and Inflammatory Cytokines in Healthy Adults. Nutrients 2014, 6, 221-230. https://doi.org/10.3390/nu6010221
Sun X, Cao Z-B, Zhang Y, Ishimi Y, Tabata I, Higuchi M. Association between Serum 25-Hydroxyvitamin D and Inflammatory Cytokines in Healthy Adults. Nutrients. 2014; 6(1):221-230. https://doi.org/10.3390/nu6010221
Chicago/Turabian StyleSun, Xiaomin, Zhen-Bo Cao, Yuping Zhang, Yoshiko Ishimi, Izumi Tabata, and Mitsuru Higuchi. 2014. "Association between Serum 25-Hydroxyvitamin D and Inflammatory Cytokines in Healthy Adults" Nutrients 6, no. 1: 221-230. https://doi.org/10.3390/nu6010221