High-Caloric and Chocolate Stimuli Processing in Healthy Humans: An Integration of Functional Imaging and Electrophysiological Findings
Abstract
:1. Introduction
2. Neuroanatomy of the Gustatory and Olfactory Pathways
2.1. The Neural Pathways Underlying Taste Perception (Gustation)
2.2. The Neural Pathways Underlying Odor Perception (Olfaction)
3. High-Caloric Food Processing and the Human Brain
3.1. fMRI Research
3.1.1. fMRI and High-Caloric Food Cue Reactivity
3.1.2. High-Caloric Food Cue Processing and Reward
| Authors | Subjects | Stimuli | Task | Results |
|---|---|---|---|---|
| Beaver et al., 2006 [26] | n = 12; 7 F/5 M | Vision: Appetizing, Disgusting, Bland, Non-Food | Passive Viewing | ↑ L OFC, ventral striatum |
| Bohon et al., 2009 [43] | n = 20; 20 F 2 Groups: Emotional vs. NonEmotional Eaters | Taste and Vision: Chocolate milkshake, tasteless solution, or no solution visual shapes (cues) | Negative vs. Neutral mood induction | ↑ L ventral ACC, thalamus across groups; ↑ L parahippocampal gyrus, ACC for emotional eaters in negative mood state during anticipation; ↑ L caudate, L pallidum, bilateral ACC during milkshake receipt in emotional eaters |
| Burger and Stice, 2012 [44] | n = 151; 74 M/77F Adolescents | Taste and Vision: Milkshake or tasteless solution Visual stimuli | Passive Viewing and Tasting | ↓ bilateral putamen, R caudate, bilateral DLPFC, mid and anterior insula to milkshake receipt in frequent consumers |
| Burger and Stice, 2011 [39] | n = 39; 39 F Adolescents | Taste and Vision: Cues associated with milkshake reward, tasteless solution, or no solution | Viewing, consumption, and anticipation of food | Positive correlation between dietary restraint scores and R OFC, DLPFC milkshake > tasteless solution activations. |
| Coletta et al., 2009 [42] | n = 19; 19 F Restrained Eaters n = 9 Unrestrained Eaters N = 10 | Vision: Highly palatable, moderately palatable, and non-food images | View images Before/after Satiety | ↓ R STG, L parahippocampal gyrus, L putamen, L middle frontal gyrus (part of DLPFC) in unrestrained eaters; ↑ cerebellum, L MFG, L DLPFC, L cingulate gyrus, R IFG, R precuneus, L parahippocampal gyrus in unrestrained during fed state |
| Evero et al., 2012 [25] | n = 30; 17 M/13 F | Vision: High energy low energy Non-food items | Passive viewing after rest and exercise | ↓ insula, ↑L precuneus activity to high-caloric foods after exercise |
| Frank et al., 2010 [27] | n = 12; 12 F | High-caloric, Low-caloric, Non-food pictures | Attend to pictures and imagine eating the food. 2 Sessions: Late follicular and Mid-late luteal phase | ↑ R NAc, R amygdala, R hippocampus in follicular compared to luteal phase; ↑ R lateral OFC, L mid cingulum in luteal relative to follicular phase |
| Frank et al., 2010 [28] | n = 12; 12 F | High-caloric, Low-caloric Non-food stimuli | Food and non-food 1-back tasks; control task | ↑ OFC, insula, occipital lobe, anterior and posterior cingulate cortex, thalamus, superior frontal lobe |
| Goldstone et al., 2009 [29] | n = 20; 10 M/10 F | Vision: High-caloric, low-caloric, non-food, and blurred images | Rate how appealing each image is during both fasted and fed states | ↑ ventral striatum, amygdala, anterior insula, medial and lateral OFC when fasted |
| Killgore and Yurgelun-Todd, 2006 [45] | n = 13; 13 F | Vision: High calorie, low calorie, non-edible (utensils) | Attempt to remember images for later recognition test | ↑ R lateral OFC with greater positive affect; ↑ medial OFC, subcallosal anterior cingulate gyrus, and posterior insula with greater negative affect |
| Killgore et al., 2003 [19] | n = 13; 13 F | Vision: High calorie, Low calorie, Non-edible (utensils) | Remember images for later recognition test | ↑ bilateral mPFC, DLPFC, thalamus, R cerebellum, middle occipital gyrus, medulla |
| Kringelbach et al., 2003 [4] | n = 10; 10 M | Taste: Chocolate milk, tomato juice, tasteless solution | Passive tasting before and after satiety | ↓ L OFC with satiety to tomato juice and chocolate milk, but no change with foods that were not consumed during the meal |
| Kroemer et al., 2013 [21] | n = 26; 13 M/13 F Fasting ghrelin levels measured | Vision: High palatable Low palatable food | Passive viewing | ↑ bilateral middle and superior occipital/temporal gyrus, fusiform, caudate, pallidum, midbrain, rolandic operculum, amygdala, thalamus, anterior cingulate gyrus, hypothalamus |
| McCabe et al., 2010 [46] | n = 45; 21M/24F Citalopram n = 15 Reboxetine n = 15 Placebo n = 15 | Taste and Vision: Liquid chocolate, liquid strawberry solution, tasteless solution; chocolate, gray control images | Rate stimulus pleasantness/unpleasantness after 7 days treatment with citalopram, reboxetine, or placebo | ↑ ventral striatum, cingulate, mid OFC ↓ ventral striatum, ventral medial OFC to chocolate with citalopram ↑ activation to chocolate with reboxetine in medial OFC/frontal pole |
| Mehta et al., 2012 [20] | n = 23; 10 M/13 F | Vision: High-caloric, Low-caloric images | Attend to stimuli during deprived and various satiated sates | ↑ bilateral amygdala positively associated with hunger scores and negatively associated with fullness in fasted state; ↑ R amygdala associated with greater hunger post-breakfast ↑ medial OFC positively associated with hunger scores in fasted state; ↑ medial OFC, L amygdala, L insula, bilateral NAc associated with food choice |
| Page et al., 2011 [30] | n = 21; 12 M/9 F | Vision: High-caloric, Low-caloric, Non-food images | Passive Viewing under euglycemic or hypoglycemic states | ↑ striatum and insula during mild hypoglycemia; ↑ activity in ACC and ventromedial PFC correlated with higher blood glucose; ↑ insula and putamen correlates with high cortisol levels |
| Passamonti et al., 2009 [31] | n = 21; 11 M/10 F | Vision: Highly appetizing Bland food images No events | Indicate image position via button press | ↑ ventral striatum, amygdala, ventral ACC |
| Piech et al., 2009 [36] | n = 8; 5 M/3 F | Vision: words Restaurant menu items High vs. Low attractiveness | Read menu item, Imagine dish in front of you, Rate how much you would like it during both hungry and satiated states | ↑ amygdala, cerebellum to high attractive items; ↑ medial and lateral OFC to high attractiveness items when hungry |
| Rolls and McCabe, 2007 [2] | n = 16; 16 F Cravers n = 8 Non-cravers n = 8 | Taste and Vision: Chocolate and condensed milk in mouth; dark and white chocolate images, grey visual cues | Rate pleasantness, intensity, and wanting for chocolate in each trial | ↑ primary taste cortex, dorsal ACC, ↑ mid OFC, ventral striatum, DLPFC to chocolate in mouth; ↑ medial OFC in cravers versus non-cravers; ↑ ACC and pregenual cingulate cortex for cravers in sight and taste of chocolate condition; ↑ mid and medial OFC, ventral striatum for cravers to sight of chocolate |
| Schur et al., 2009 [32] | n = 10; 10 F | Vision: Fattening, non-fattening, and non-food object images | Remember what images were presented | ↑ midbrain including ventral tegmental area, hypothalamus, L amygdala, L DLPFC, L OFC, R insula, striatum, thalamus, areas 17 and 18 of occipital lobe for fattening > non-food contrast; ↑ brainstem, R hypothalamus, L amygdala, R inferior frontal gyrus, insula, striatum, thalamus |
| Siep et al., 2009 [3] | n = 12; 12 F | Vision: High-caloric, Low-caloric, and Neutral images | Indicate palatability of foods and vividly imagine their tastes, color of neutral objects, or orientations of bars during food deprived and satiated states | Reduced inhibition of L medial PFC; ↑ fusiform, R medial OFC, R insula, L caudate putamen, PCC during hunger |
| Small et al., 2005 [5] | n = 11 | Smell: Butanol, farnesol, lavender, and chocolate odors | Passive perception of odors Pleasantness/intensity ratings after each run | ↑ medial OFC, perigenual cingulate to chocolate during retronasal administration; ↑ thalamus, R caudolateral OFC, R hippocampus, perisylvian and insular cortices for orthonasal administration |
| Smeets et al., 2006 [47] | n = 24; 12M/12 F | Taste: Chocolate milk, Eating solid chocolate | Taste chocolate milk during fasted and satiated states Indicate motivation to eat chocolate during scans | ↑ L ventral striatum, L precentral gyrus, DLPFC, L dorsal striatum, anterior insula, OFC, medial OFC; ↓ inferior and superior parietal lobules, medial PFC for satiety in men; ↑ precentral gyrus, R superior temporal gyrus, ventral striatum; ↓ hypothalamus and amygdala for satiety in females |
3.2. Event-Related Potential Research (ERPs)
| Authors | Subjects | Stimuli | Task | Results |
|---|---|---|---|---|
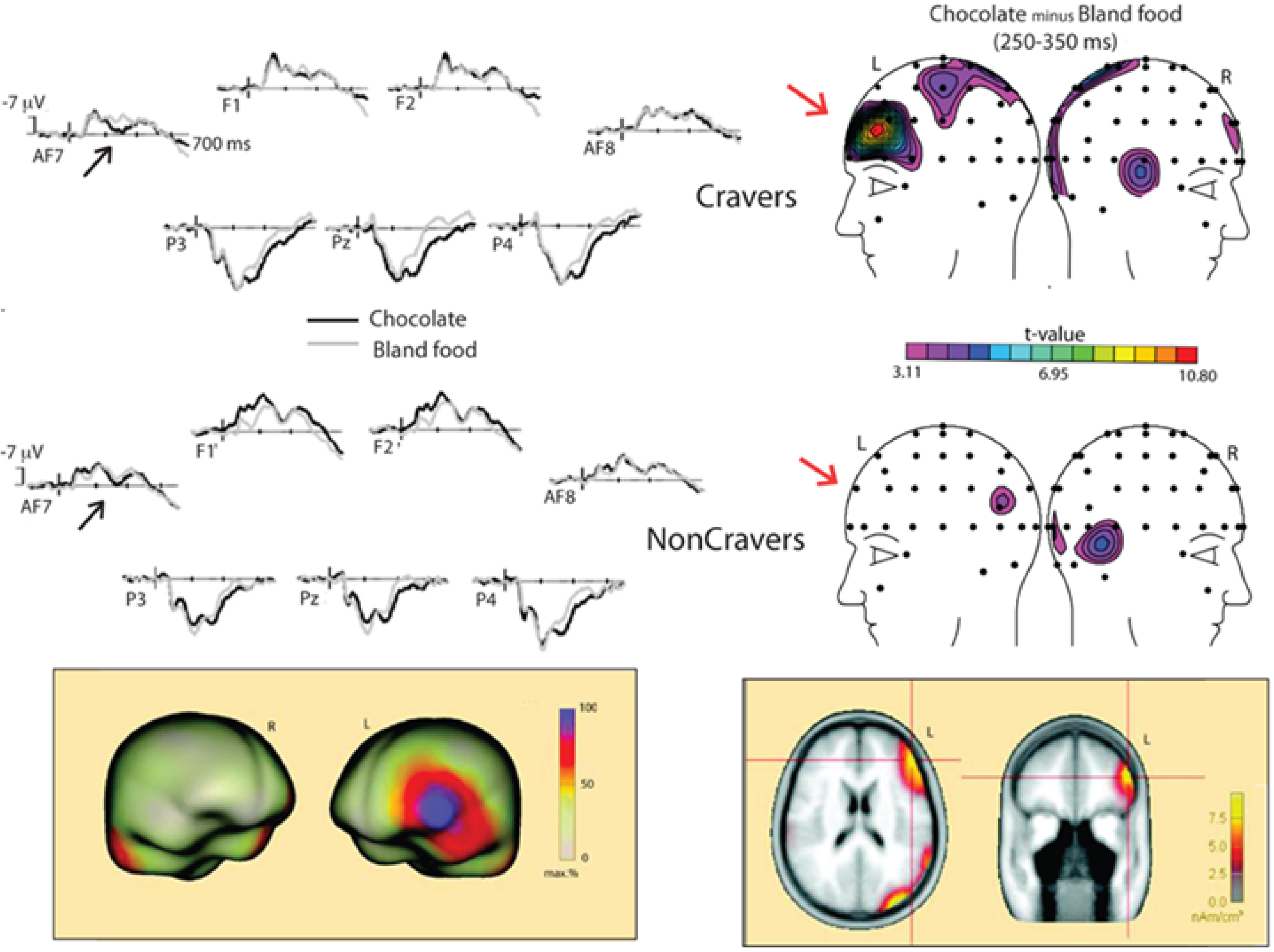
| Asmaro et al., 2012 [1] | n = 26; 26 F Chocolate Cravers (n = 14) NonCravers (n = 12) | Vision: Chocolate and bland food images | Passive viewing with satiety between sessions | EAP (250–350 ms); LPP (360–560 ms); AN (100–250 ms) |
| Blechert et al., 2010 [56] | n = 40; 40 F Restrained Eaters (n = 19) vs. Non-restrained Eaters (n = 21) | Vision: High-caloric, pleasant, neutral, unpleasant pictures | Passive viewing of available and unavailable foods | ↓ LPP (300–700 ms) to available food cues in restrained eaters |
| Gable and Harmon-Jones, 2010 [54] | n = 30; 19 M/11 F | Vision: Appetitive (desserts) neutral (rocks), Navon stimuli | View appetitive and neutral pictures; decide whether Navon stimuli contained either the letter T or letter H | ↑ LPP (500–1000 ms) to appetitive cues |
| Kemmotsu and Murphy, 2006 [57] | 17 female restrained eaters 18 female unrestrained eaters | Smell: Chocolate and non-food odors | Ignore or attend to chocolate and non-food odors | ↑ N1P2 in unrestrained to chocolate odor in ignore condition; ↑ N1P3 in unrestrained to chocolate odor for attend relative to ignore condition |
| Lietti et al., 2012 [52] | n = 21; 10 M/11 F | Vision: High energy, low energy, and non-food stimuli | Decide whether stimulus is a food or non-food item | No difference is VEP topographies when high energy foods repeatedly presented; Source localization indicates generators in prefrontal and middle temporal cortices |
| Ohla et al., 2012 [51] | n = 14; 9 M/5 F | Vision, Taste: High-caloric, low-caloric, and non-food images; taste stimuli delivered by electrical stimulation of tongue | Categorize food and non-food images; evaluate taste pleasantness and intensity | ↑ source strength in R insular gyrus, FOP transition, L FOP, L middle frontal gyrus, R parahippocampal gyrus (92–174 ms) when high-caloric images preceded taste; ↑ medial orbitofrontal gyrus, ACC, left superior and middle frontal gyrus, parahippocampal, fusiform (176–236 ms) when high-caloric images preceded taste; ↑ R middle frontal gyrus, R insula, parietal operculum, postcentral gyrus, R occipital gyrus (357–500 ms) when high-caloric viewed before taste stimulation |
| Stockburger et al., 2009 [55] | n = 32; 16 M/16 F | Vision: Appetizing food, pleasant, unpleasant, neutral, and flower images | View images in food deprived and satiated states | ↑ positivity to food images over posterior electrode sites (60–300 ms); ↑ negative potential to food at occipito-temporal sensors (more left-sided) and a centro-frontal positivity during deprived state (300–350 ms); ↑ positive potential at parietal sensors (polarity reversed at frontal channels) to food during deprived state (450–600 ms) |
| Stockburger et al., 2008 [53] | n = 16; 16 M | Vision: Appetizing food, Pleasant, Unpleasant, Neutral images | View images during food deprived and satiated states | ↑ posterior positivity to food (170–210 ms and 270–310 ms) during hungry state |
| Toepel et al., 2012 [49] | n = 24; 12 M/12 F | Vision: High fat, Low fat Non-food images | Decide whether stimulus was a food or non-food item | ↑ VEP for women, which interacted with BMI values (170–213 ms); Source localization found potential generators in ventromedial PFC, posterior middle temporal cortex, superior parietal lobe of left hemisphere; ventromedial PFC, anterior temporal cortex, and inferior parietal lobe |
| Toepel, et al., 2009 [50] | n = 24; 12 M/12 F | Vision: High fatLow fat Non-food images. | Decide whether stimulus was a food or non-food item | ↑ VEP to high fat food images between 166–230 and 309–371 ms; Source localization found lateral occipital, superior temporal, left postcentral gyrus (166–230 ms); Source localization found greater L occipito-temporal cortex, L inferior parietal lobule, L dorsal frontal, R ventromedial PFC, R dorsal and lateral PFC (309–371 ms) |
4. Research with Chocolate as a Target Stimulus
4.1. Chocolate Cue Reactivity and fMRI
4.2. Chocolate Cue Reactivity and ERPs
5. Factors Involved in the Modulation of Food-Elicited Neural Reponses
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Asmaro, D.; Jaspers-Fayer, F.; Sramko, V.; Taake, I.; Carolan, P.; Liotti, M. Spatiotemporal dynamics of the hedonic processing of chocolate images in individuals with and without trait chocolate craving. Appetite 2012, 58, 790–799. [Google Scholar] [CrossRef]
- Rolls, E.T.; McCabe, C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur. J. Neurosci. 2007, 26, 1067–1076. [Google Scholar] [CrossRef]
- Siep, N.; Roefs, A.; Roebroeck, A.; Havermans, R.; Bonte, M.L.; Jansen, A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav. Brain Res. 2009, 198, 149–158. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; O’Doherty, J.; Rolls, E.T.; Andrews, C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb. Cortex 2003, 13, 1064–1071. [Google Scholar] [CrossRef]
- Small, D.M.; Gerber, J.C.; Mak, Y.E.; Hummel, T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 2005, 47, 593–605. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef]
- Rolls, E.T. Brain mechanisms underlying flavour and appetite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1123–1136. [Google Scholar] [CrossRef]
- Galindo, M.M.; Schneider, N.Y.; Stähler, F.; Töle, J.; Meyerhof, W. Taste preferences. Prog. Mol. Biol. Transl. Sci. 2012, 108, 383–426. [Google Scholar] [CrossRef]
- De Araujo, I.E.; Rolls, E.T.; Kringelbach, M.L.; McGlone, F.; Phillips, N. Taste-olfactory convergence, and the representation of the pleasantness of flavor, in the human brain. Eur. J. Neurosci. 2003, 18, 2059–2068. [Google Scholar] [CrossRef]
- Simon, S.A.; de Araujo, I.E.; Gutierrez, R.; Nicolelis, M.A. The neural mechanisms of gustation: A distributed processing code. Nat. Rev. Neurosci. 2006, 7, 890–901. [Google Scholar] [CrossRef]
- Frank, S.; Kullmann, S.; Veit, R. Food related processes in the insular cortex. Front. Hum. Neurosci. 2013, 7, 499. [Google Scholar]
- Garcia-Garcia, I.; Narberhaus, A.; Marques-Iturria, I.; Garolera, M.; Radoi, A.; Segura, B.; Pueyo, R.; Ariza, M.; Jurado, M.A. Neural responses to visual food cues: Insights from functional magnetic resonance imaging. Eur. Eat. Disord. Rev. 2013, 21, 89–98. [Google Scholar] [CrossRef]
- Valentin, V.V.; Dickinson, A.; O’Doherty, J.P. Determining the neural substrates of goal-directed learning in the human brain. J. Neurosci. 2007, 27, 4019–4026. [Google Scholar]
- Berridge, K.C. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef]
- Kaupp, U.B. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat. Rev. Neurosci. 2010, 11, 188–200. [Google Scholar]
- Huart, C.; Rombaux, P.; Hummel, T. Plasticity of the human olfactory system: The olfactory bulb. Molecules 2013, 18, 11586–11600. [Google Scholar]
- Kadohisa, M. Effects of odor on emotion, with implications. Front. Syst. Neurosci. 2013, 7, 66. [Google Scholar] [CrossRef]
- Kemps, E.; Tiggemann, M.; Bettany, S. Non-food odorants reduce chocolate cravings. Appetite 2012, 58, 1087–1090. [Google Scholar] [CrossRef]
- Killgore, W.D.; Young, A.D.; Femia, L.A.; Bogorodzki, P.; Rogowska, J.; Yurgelun-Todd, D.A. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003, 19, 1381–1394. [Google Scholar] [CrossRef]
- Mehta, S.; Melhorn, S.J.; Smeraglio, A.; Tyagi, V.; Grabowski, T.; Schwartz, M.W.; Schur, E.A. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am. J. Clin. Nutr. 2012, 96, 989–999. [Google Scholar] [CrossRef]
- Kroemer, N.B.; Krebs, L.; Kobiella, A.; Grimm, O.; Pilhatsch, M.; Bidlingmaier, M.; Zimmermann, U.S.; Smolka, M.N. Fasting levels of ghrelin covary with the brain response to food pictures. Addict. Biol. 2013, 5, 855–862. [Google Scholar]
- Lawrence, N.S.; Hinton, E.C.; Parkinson, J.A.; Lawrence, A.D. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage 2012, 63, 415–422. [Google Scholar] [CrossRef]
- Demos, K.E.; Heatherton, T.F.; Kelley, W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012, 32, 5549–5552. [Google Scholar] [CrossRef]
- Tang, D.W.; Fellows, L.K.; Small, D.M.; Dagher, A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol. Behav. 2012, 106, 317–324. [Google Scholar] [CrossRef]
- Evero, N.; Hackett, L.C.; Clark, R.D.; Phelan, S.; Hagobian, T.A. Aerobic exercisereduces neuronal responses in food reward brain regions. J. Appl. Physiol. 2012, 112, 1612–1619. [Google Scholar] [CrossRef]
- Beaver, J.D.; Lawrence, A.D.; van Ditzhuijzen, J.; Davis, M.H.; Woods, A.; Calder, A.J. Individual differences in reward drive predict neural responses to images of food. J. Neurosci. 2006, 26, 5160–5166. [Google Scholar]
- Frank, T.C.; Kim, G.L.; Krzemien, A.; van Vugt, D.A. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. 2010, 1363, 81–92. [Google Scholar] [CrossRef]
- Frank, S.; Laharnar, N.; Kullmann, S.; Veit, R.; Canova, C.; Hegner, Y.L.; Fritsche, A.; Preissl, H. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res. 2010, 1350, 159–166. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Prechtl de Hernandez, C.G.; Beaver, J.D.; Muhammed, K.; Croese, C.; Bell, G.; Durighel, G.; Hughes, E.; Waldman, A.D.; Frost, G.; et al. Fasting biases brain reward systems towards high-calorie foods. Eur. J. Neurosci. 2009, 30, 1625–1635. [Google Scholar] [CrossRef]
- Page, K.A.; Seo, D.; Belfort-DeAguiar, R.; Lacadie, C.; Dzuira, J.; Naik, S.; Amarnath, S.; Constable, R.T.; Sherwin, R.S.; Sinha, R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J. Clin. Investig. 2011, 121, 4161–4169. [Google Scholar] [CrossRef]
- Passamonti, L.; Rowe, J.B.; Schwarzbauer, C.; Ewbank, M.P.; von dem Hagen, E.; Calder, A.J. Personality predicts the brain’s response to viewing appetizing foods: The neural basis of a risk factor for overeating. J. Neurosci. 2009, 29, 43–51. [Google Scholar] [CrossRef]
- Schur, E.A.; Kleinhans, N.M.; Goldberg, J.; Buchwald, D.; Schwartz, M.W.; Maravilla, K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int. J. Obes. 2009, 33, 653–661. [Google Scholar]
- Gearhardt, A.N.; Yokum, S.; Orr, P.T.; Stice, E.; Corbin, W.R.; Brownell, K.D. Neural correlates of food addiction. Arch. Gen. Psychiatry 2011, 68, 808–816. [Google Scholar] [CrossRef]
- Zhang, Y.; von Deneen, K.M.; Tian, J.; Gold, M.S.; Liu, Y. Food addiction and neuroimaging. Curr. Pharm. Des. 2011, 17, 1149–1157. [Google Scholar] [CrossRef]
- Liu, X.; Hairston, J.; Schrier, M.; Fan, J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011, 35, 1219–1236. [Google Scholar] [CrossRef]
- Piech, R.M.; Lewis, J.; Parkinson, C.H.; Owen, A.M.; Roberts, A.C.; Downing, P.E.; Parkinson, J.A. Neural correlates of appetite and hunger-related evaluative judgments. PLoS One 2009, 4, e6581. [Google Scholar]
- Cauda, F.; Cavanna, A.E.; D’agata, F.; Sacco, K.; Duca, S.; Geminiani, G.C. Functional connectivity and coactivation of the nucleus accumbens: A combined functional connectivity and structure-based meta-analysis. J. Cogn. Neurosci. 2011, 23, 2864–2877. [Google Scholar] [CrossRef]
- Krebs, R.M.; Boehler, C.N.; Egner, T.; Waldorff, M.G. The neural underpinnings of how reward associations can both guide and misguide attention. J. Neurosci. 2011, 31, 9752–9759. [Google Scholar] [CrossRef] [Green Version]
- Burger, K.S.; Stice, E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage 2011, 55, 233–239. [Google Scholar] [CrossRef]
- Killgore, W.D.; Yurgelun-Todd, D.A. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport 2010, 21, 354–358. [Google Scholar] [CrossRef]
- Martin-Soelch, C.; Linthicum, J.; Ernst, M. Appetitive conditioning: Neural bases and implications for psychopathology. Neurosci. Biobehav. Rev. 2007, 31, 426–440. [Google Scholar]
- Coletta, M.; Platek, S.; Mohamed, F.B.; van Steenburgh, J.J.; Green, D.; Lowe, M.R. Brain activation in restrained and unrestrained eaters: An fMRI study. J. Abnorm. Psychol. 2009, 118, 598–609. [Google Scholar] [CrossRef]
- Bohon, C.; Stice, E.; Spoor, S. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: A functional magnetic resonance imaging study. Int. J. Eat. Disord. 2009, 42, 210–221. [Google Scholar] [CrossRef]
- Burger, K.S.; Stice, E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. Am. J. Clin. Nutr. 2012, 95, 810–817. [Google Scholar] [CrossRef]
- Killgore, W.D.; Yurgelun-Todd, D.A. Affect modulates appetite-related brain activity to images of food. Int. J. Eat. Disord. 2006, 39, 357–363. [Google Scholar] [CrossRef]
- McCabe, C.; Mishor, Z.; Cowen, P.J.; Harmer, C.J. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol. Psychiatry 2010, 67, 439–445. [Google Scholar] [CrossRef]
- Smeets, P.A.; de Graaf, C.; Stafleu, A.; van Osch, M.J.; Nievelstein, R.A.; van der Grond, J. Effect of satiety on brain activation during chocolate tasting in men and women. Am. J. Clin. Nutr. 2006, 83, 1297–1305. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Toepel, U.; Knebel, J.F.; Hudry, J.; le Coutre, J.; Murray, M.M. Gender and weight shape brain dynamics during food viewing. PLoS One 2012, 7, e36778. [Google Scholar]
- Toepel, U.; Knebel, J.F.; Hudry, J.; le Coutre, J.; Murray, M.M. The brain tracks the energetic value in food images. Neuroimage 2009, 44, 967–974. [Google Scholar]
- Ohla, K.; Toepel, U.; le Coutre, J.; Hudry, J. Visual-gustatory interaction: Orbitofrontal andinsular cortices mediate the effect of high-calorie visual food cues on taste pleasantness. PLoS One 2012, 7, e32434. [Google Scholar]
- Lietti, C.V.; Murray, M.M.; Hudry, J.; le Coutre, J.; Toepel, U. The role of energetic value in dynamic brain response adaptation during repeated food image viewing. Appetite 2012, 58, 11–18. [Google Scholar] [CrossRef]
- Stockburger, J.; Weike, A.I.; Hamm, A.O.; Schupp, H.T. Deprivation selectively modulates brain potentials to food. Behav. Neurosci. 2008, 122, 936–942. [Google Scholar] [CrossRef]
- Gable, P.A.; Harmon-Jones, E. Late positive potential to appetitive stimuli and localattentional bias. Emotion 2010, 10, 441–446. [Google Scholar] [CrossRef]
- Stockburger, J.; Schmälzle, R.; Flaisch, T.; Bublatzky, F.; Schupp, H.T. The impact of hunger on food cue processing: An event-related brain potential study. Neuroimage 2009, 47, 1819–1829. [Google Scholar]
- Blechert, J.; Feige, B.; Hajcak, G.; Tuschen-Caffier, B. To eat or not to eat? Availability of food modulates the electrocortical response to food pictures in restrained eaters. Appetite 2010, 54, 262–268. [Google Scholar] [CrossRef]
- Kemmotsu, N.; Murphy, C. Restrained eaters show altered brain response to food odor. Physiol. Behav. 2006, 87, 323–329. [Google Scholar] [CrossRef]
- Small, D.M.; Zatorre, R.J.; Dagher, A.; Evans, A.C.; Jones-Gotman, M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 2001, 124, 1720–1733. [Google Scholar] [CrossRef]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharm. 2006, 47, S215–S220. [Google Scholar] [CrossRef]
- Koeneke, S.; Pedroni, A.F.; Dieckmann, A.; Bosch, V.; Jäncke, L. Individual preferences modulate incentive values: Evidence from functional MRI. Behav. Brain Funct. 2008, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef]
- Macht, M.; Mueller, J. Immediate effects of chocolate on experimentally induced mood states. Appetite 2007, 49, 667–674. [Google Scholar]
- Camfield, D.A.; Scholey, A.; Pipingas, A.; Silberstein, R.; Kras, M.; Nolidin, K.; Wesnes, K.; Pase, M.; Stough, C. Steady state visually evoked potential (SSVEP) topography changes associated with flavanol consumption. Physiol. Behav. 2012, 105, 948–957. [Google Scholar] [CrossRef]
- Asmaro, D.; Carolan, P.; Liotti, M. Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addict. Behav. 2014, 39, 114–121. [Google Scholar] [CrossRef]
- Grimm, O.; Jacob, M.J.; Kroemer, N.B.; Krebs, L.; Vollstädt-Klein, S.; Kobiella, A.; Wolfensteller, U.; Smolka, M.L. The personality trait self-directedness predicts the amygdala’s reaction to appetizing cues in fMRI. Appetite 2012, 58, 1023–1029. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asmaro, D.; Liotti, M. High-Caloric and Chocolate Stimuli Processing in Healthy Humans: An Integration of Functional Imaging and Electrophysiological Findings. Nutrients 2014, 6, 319-341. https://doi.org/10.3390/nu6010319
Asmaro D, Liotti M. High-Caloric and Chocolate Stimuli Processing in Healthy Humans: An Integration of Functional Imaging and Electrophysiological Findings. Nutrients. 2014; 6(1):319-341. https://doi.org/10.3390/nu6010319
Chicago/Turabian StyleAsmaro, Deyar, and Mario Liotti. 2014. "High-Caloric and Chocolate Stimuli Processing in Healthy Humans: An Integration of Functional Imaging and Electrophysiological Findings" Nutrients 6, no. 1: 319-341. https://doi.org/10.3390/nu6010319




