Dietary, Lifestyle and Socio-Economic Correlates of Overweight, Obesity and Central Adiposity in Lebanese Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Anthropometric Measurements
2.3. Definitions of Overweight and Obesity
- -
- Adult cut-off values for subjects aged 16–19 years (WC > 94 cm for males and >80 cm for females).
- -
- Cut-off value of WC ≥ 90th percentile for sex and age (or adult cut-offs if lower) for subjects aged 6 to 15 years old. As national WC percentiles are lacking in Lebanon, the WC percentiles for children and adolescents as developed by Fernandez et al. (2004) were used [26].
2.4. Dietary Intake and Physical Activity Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Sample
| Variables | Age Group (years) | Total (1) | p-Value (2) | |
|---|---|---|---|---|
| 6–11 (n = 370) | 12–19 (n = 498) | (n = 868) | ||
| Socio-Demographic characteristics n (%) | ||||
| Gender | ||||
| Male | 191 (51.6) | 248 (49.8) | 439 (50.6) | 0.595 |
| Female | 179 (48.4) | 250 (50.2) | 429 (49.4) | |
| Governorates | ||||
| Capital (Beirut) | 26 (7.0) | 36 (7.2) | 62 (7.1) | 0.909 |
| Other governorates | 344 (93.0) | 462 (92.8) | 806 (92.9) | |
| Father’s Education | ||||
| Primary or less | 105 (28.8) | 156 (31.7) | 261 (30.5) | 0.571 |
| Intermediate | 112 (30.8) | 153 (31.1) | 265 (31.0) | |
| High school and above | 147 (40.4) | 183 (37.2) | 330 (38.6) | |
| Mother’s Education | ||||
| Primary or less | 76 (23.0) | 112 (26.3) | 188 (24.9) | 0.587 |
| Intermediate | 97 (29.4) | 121 (28.4) | 218 (28.8) | |
| High school and above | 157 (47.6) | 193 (45.3) | 350 (46.3) | |
| Mother’s working status | ||||
| Not working | 291 (80.6) | 350 (70.4) | 641 (74.7) | 0.001 |
| Working | 70 (19.4) | 147 (29.6) | 217 (25.3) | |
| Parental Obesity (3) | ||||
| No | 218 (71.5) | 171 (66.0) | 389 (69.0) | 0.163 |
| Yes | 87 (28.5) | 88 (34.0) | 175 (31.0) | |
| Crowding Index | ||||
| <1 person/room | 66 (18.0) | 92 (18.5) | 158 (18.3) | 0.858 |
| ≥1 person/room | 300 (82.0) | 405 (81.5) | 705 (81.7) | |
| Lifestyle characteristics n (%) | ||||
| Breakfast consumption (per week) | ||||
| Never | 11 (3.0) | 36 (7.2) | 47 (5.4) | <0.001 |
| Sometimes | 39 (10.6) | 116 (23.3) | 155 (17.9) | |
| Daily | 319 (86.4) | 346 (69.5) | 665 (76.7) | |
| Frequency of eating outside home (per week) | ||||
| ≤1 time | 205 (55.6) | 207 (41.6) | 412 (47.5) | <0.001 |
| >1 time | 164 (44.4) | 291 (58.4) | 455 (52.5) | |
| Physical Activity (4) | ||||
| Low | 80 (21.7) | 193 (41.2) | 273 (32.6) | <0.001 |
| Moderate | 53 (14.4) | 119 (25.4) | 172 (20.5) | |
| High | 235 (63.9) | 157 (33.5) | 392 (46.8) | |
| Sedentary time (h/day) Mean ± SD | 8.72 ± 2.77 | 10.09 ± 2.94 | 9.51 ± 2.95 | <0.001 |
| Anthropometric characteristics | ||||
| Weight (kg) | ||||
| Mean ± SD | 35.01 ± 12.55 | 60.77 ± 15.61 | 49.81 ± 19.21 | <0.001 |
| 10th percentile | 22.15 | 42.67 | 25.88 | |
| 50th percentile | 32.45 | 59 | 48.75 | |
| 90th percentile | 50.81 | 80.81 | 74.52 | |
| Height (cm) | ||||
| Mean ± SD | 135.63 ± 12.26 | 164.17 ± 10.07 | 152.02 ± 17.93 | <0.001 |
| 10th percentile | 119.75 | 152 | 126 | |
| 50th percentile | 136 | 164 | 154 | |
| 90th percentile | 152 | 177.5 | 174.05 | |
| BMI (kg/m2) | <0.001 | |||
| Mean ± SD | 18.53 ± 3.99 | 22.32 ± 4.37 | 20.71 ± 4.61 | |
| 10th percentile | 14.65 | 17.47 | 15.6 | |
| 50th percentile | 17.58 | 21.58 | 20.08 | |
| 90th percentile | 23.56 | 28.17 | 26.8 | |
| WC (cm) | ||||
| Mean ± SD | 63.77 ± 10.75 | 74.93 ± 10.97 | 70.18 ± 12.19 | <0.001 |
| 10th percentile | 52.75 | 62.73 | 56 | |
| 50th percentile | 61.5 | 73.2 | 69 | |
| 90th percentile | 78.5 | 91 | 86.05 | |
| WHtR | ||||
| Mean ± SD | 0.47 ± 0.06 | 0.46 ± 0.06 | 0.46 ± 0.06 | 0.002 |
| 10th percentile | 0.41 | 0.39 | 0.4 | |
| 50th percentile | 0.46 | 0.44 | 0.45 | |
| 90th percentile | 0.55 | 0.54 | 0.55 | |
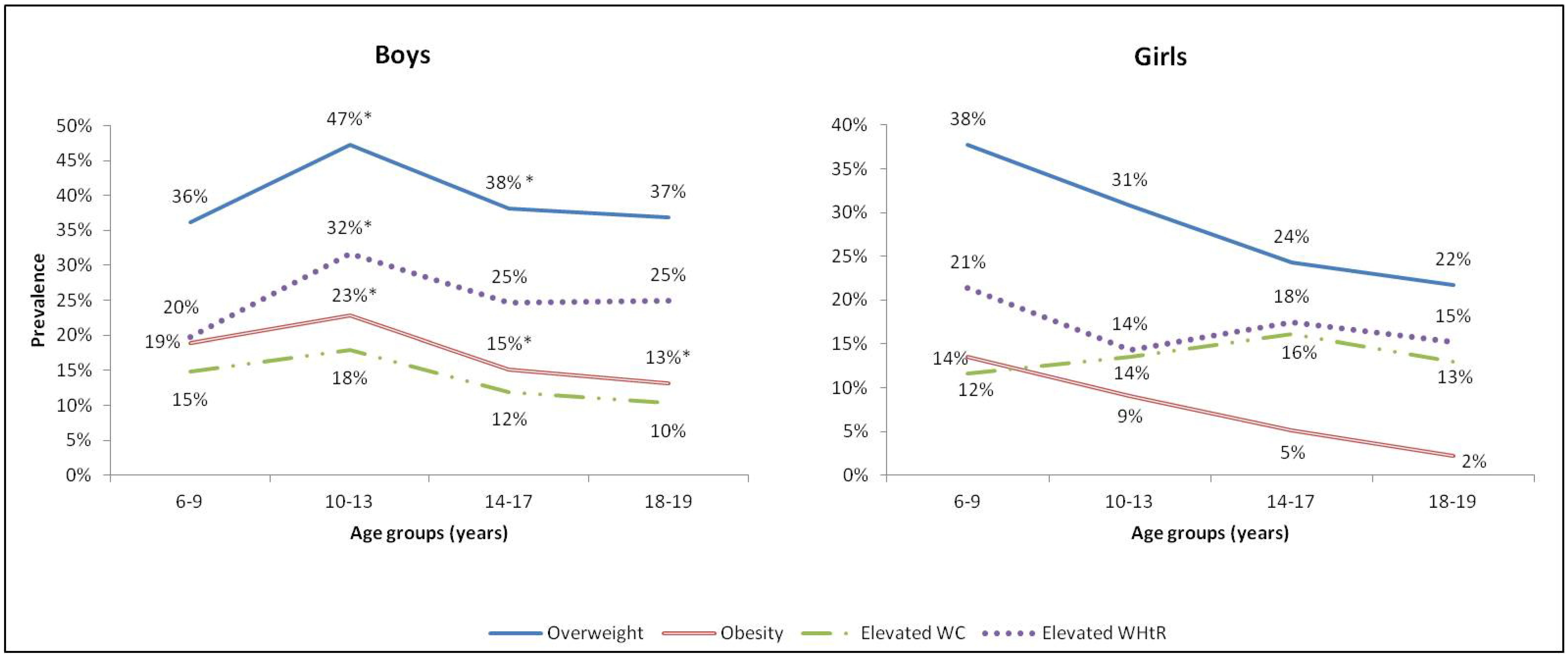
3.2. Prevalence of Overweight, Obesity and Abdominal Obesity
| Variables | Age Groups (years) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6–11 | 12–19 | 6–19 | |||||||
| n | % | (95% CI) | n | % | (95% CI) | n | % | (95% CI) | |
| Male | |||||||||
| Overweight (1) | 81 | 42.4 | (36–50) | 94 | 37.9 a | (32–44) | 175 | 39.9 a | (35–44) |
| Obesity (1) | 39 | 20.4 | (15–27) | 40 | 16.1 b | (12–21) | 79 | 18.0 b | (15–22) |
| Elevated WC (2) | 30 | 15.7 | (11–22) | 32 | 12.9 | (9–18) | 62 | 14.1 | (11–18) |
| Elevated WHtR (3) | 46 | 24.1 | (19–31) | 65 | 26.2 c | (21–32) | 111 | 25.3 c | (21–30) |
| Female | |||||||||
| Overweight (1) | 67 | 37.9 | (31–45) | 59 | 23.7 a | (19–29) | 126 | 29.6 a | (25–34) |
| Obesity (1>) | 24 | 13.6 | (9–19) | 11 | 4.4 b | (2–8) | 35 | 8.2 b | (6–11) |
| Elevated WC (2) | 21 | 11.8 | (8–17) | 38 | 15.2 | (11–20) | 59 | 13.8 | (11–17) |
| Elevated WHtR (3) | 35 | 19.7 | (14–26) | 39 | 15.6 c | (12–21) | 74 | 17.3 c | (14–21) |
| Both Genders | |||||||||
| Overweight (1) | 148 | 40.2 | (35–45) | 153 | 30.8 | (27–35) | 301 | 34.8 | (32–38) |
| Obesity (1) | 63 | 17.1 | (14–21) | 51 | 10.3 | (8–13) | 114 | 13.2 | (11–16) |
| Elevated WC (2) | 51 | 13.8 | (10–17) | 70 | 14.1 | (11–17) | 121 | 14.0 | (11–16) |
| Elevated WHtR (3) | 81 | 22.0 | (18–27) | 104 | 20.9 | (17–24) | 185 | 21.3 | (18–24) |
3.3. Dietary Intake
| Dietary Variables | Age Group (years) | Total | ||||
|---|---|---|---|---|---|---|
| 6–11 (n = 370) | 12–19 (n = 498) | 6–19 (n = 868) | ||||
| Energy (kcal ± SE ) | 1736.48 ± 36.12 a | 2255.85 ± 51.60 a | 2033.70 ± 34.47 | |||
| Mean % Daily Energy Intake ± SE | ||||||
| Carbohydrates | 52.01 ± 0.51 | 51.05 ± 0.48 | 51.46 ± 0.35 | |||
| Protein | 13.11 ± 0.18 | 13.53 ± 0.23 | 13.35 ± 0.15 | |||
| Fat | 35.86 ± 0.47 | 36.24 ± 0.43 | 36.058 ± 0.32 | |||
| Breads and Cereals | 36.92 | ±0.89 b | 32.67 | ±0.77 b | 34.49 | ±0.58 |
| Milk and Dairies | 8.90 | ±0.52 c | 6.47 | ±0.37 c | 7.51 | ±0.31 |
| Meat and Equivalent | 10.22 | ±0.57 | 10.15 | ±0.57 | 10.18 | ±0.40 |
| Legumes and Nuts | 2.05 | ±0.34 d | 3.28 | ±0.38 d | 2.76 | ±0.27 |
| Fruits and Vegetables | 5.35 | ±0.33 | 5.42 | ±0.33 | 5.39 | ±0.24 |
| Added Fats and Oils | 7.58 | ±0.47 | 8.42 | ±0.42 | 8.06 | ±0.31 |
| Fast Food | 11.35 | ±0.69 e | 17.27 | ±0.92 e | 14.74 | ±0.61 |
| Sugar and Sweets | 10.81 | ±0.63 | 9.65 | ±0.60 | 10.15 | ±0.44 |
| Sugar Sweetened Beverages | 6.52 | ±0.45 | 6.45 | ±0.32 | 6.48 | ±0.26 |
3.4. Factors Associated with Overweigh, Obesity and Abdominal Obesity
| Variables | 6–11 Years | |||
|---|---|---|---|---|
| (n = 370) | ||||
| Overweight 1 | Obesity 1 | Elevated WHtR | Elevated WC | |
| Odds Ratio [95% CI] | ||||
| Socio-Demographic Factors | ||||
| Age (years) | 1.17 [1.00–1.36] | 1.17 [0.91–1.37] | 1.14 [0.97–1.34] | 1.22 [0.96–1.54] |
| Sex | ||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.17 [0.69–1.97] | 1.92 [0.94–3.90] | 1.47 [0.84–2.56] | 2.01 [0.90–4.48] |
| Place of Residence | ||||
| Beirut (Capital) | 1.00 | 1.00 | 1.00 | 1.00 |
| Other Governorates | 0.32 [0.10–0.98] | 0.21 [0.06–0.71] | 0.39 [0.14–1.05] | 0.16 [0.04–0.56] |
| Father’s Education 2 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.14 [0.57–2.29] | 0.83 [0.35–1.96] | 0.98 [0.46–2.07] | 1.12 [0.43–2.92] |
| High | 0.52 [0.24–1.11] | 0.32 [0.11–0.91] | 0.73 [0.33–1.62] | 0.46 [0.14–1.48] |
| Mother’s Education 2 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.98 [0.90–4.33] | 1.59 [0.60–4.18] | 0.66 [0.30–1.47] | 0.68 [0.23–1.95] |
| High | 2.45 [1.13–5.31] | 1.36 [0.52–3.59] | 0.68 [0.31–1.45] | 0.80 [0.29–2.20] |
| Mother’s Working Status | ||||
| Not Working | 1.00 | 1.00 | 1.00 | 1.00 |
| Working | 1.06 [0.55–2.03] | 2.60 [1.18–5.70] | 2.27 [1.19–4.33] | 1.47 [0.60–3.63] |
| Crowding Index | ||||
| <1 person/room | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥1 person/room | 1.19 [0.59–2.40] | 1.04 [0.40–2.70] | 0.64 [0.31–1.31] | 0.53 [0.20–1.40] |
| Parental Obesity | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.72 [0.97–3.04] | 2.67 [1.34–5.31] | 2.10 [1.18–3.72] | 2.46 [1.15–5.23] |
| Lifestyle and Dietary Factors | ||||
| Physical Activity 3 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.60 [0.91–2.81 | 1.78 [0.90–3.52] | 0.99 [0.52–1.86] | 1.43 [0.71–2.89] |
| High | 0.86 [0.56–1.33] | 0.75 [0.42–1.33] | 0.70 [0.43–1.14 | 0.68 [0.38–1.22] |
| Sedentary Time (h/day) | 1.02 [0.93–1.12] | 1.10 [0.97–1.25] | 1.05 [0.93–1.19] | 1.08 [0.94–1.25] |
| Daily Breakfast Consumption | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.20 [0.05–0.84] | 0.07 [0.01–0.30] | 0.32 [0.07–1.32] | 0.25 [0.05–1.25] |
| Frequency of Eating Out | ||||
| ≤1 time/week | 1.00 | 1.00 | 1.00 | 1.00 |
| >1 time/week | 1.01 [0.62–1.65] | 0.96 [0.48–1.93] | 1.18 [0.65–2.14] | 1.75 [0.82–3.70] |
| Total Daily Energy Intake (kcal) 2 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.21 [0.70–2.10] | 1.19 [0.54–2.65] | 1.43 [0.73–2.78] | 1.13 [0.48–2.66] |
| High | 1.44 [0.71–2.90] | 1.10 [0.52–3.74] | 1.13 [0.47–2.71] | 1.54 [0.54–4.43] |
| Bread and Cereals 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.19 [0.67–2.12] | 0.76 [0.32–1.79] | 0.72 [0.35–1.46] | 0.51 [0.20–1.32] |
| High | 1.01 [0.53–1.86] | 1.36 [0.59–3.14] | 0.97 [0.46–2.01] | 1.13 [0.46–2.73] |
| Milk and Dairies 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.92 [0.50–1.69] | 0.99 [0.43–2.30] | 1.07 [0.52–2.22] | 0.53 [0.21–1.32] |
| High | 1.16 [0.62–2.16] | 0.64 [0.26–1.56] | 0.66 [0.30–1.43] | 0.35 [0.13–0.91] |
| Meat and Equivalent 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.86 [0.48–1.53] | 1.19 [0.54–2.61] | 1.24 [0.63–2.44] | 1.38 [0.56–3.18] |
| High | 0.71 [0.34–1.47] | 0.64 [0.21–1.94] | 1.32 [0.53–3.29] | 1.26 [0.38–4.17] |
| Legumes and Nuts 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.08 [0.66–1.76] | 0.65 [0.34–1.23] | 0.74 [0.41–1.33] | 0.86 [0.42–1.74] |
| High | 0.90 [0.51–1.58] | 0.68 [0.33–1.42] | 0.93 [0.49–1.78] | 1.17 [0.55–2.51] |
| Fruits and Vegetables 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.55 [0.86–2.79] | 2.24 [0.99–5.08] | 1.08 [0.49–2.37] | 2.01 [0.83–4.86] |
| High | 0.91 [0.50–1.67] | 1.11 [0.45–2.69] | 0.99 [0.48–2.03] | 1.11 [0.42–2.90] |
| Added Fats and Oils 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.73 [0.40–1.32] | 0.36 [0.17–0.86] | 0.42 [0.20–0.88] | 0.79 [0.32–1.93] |
| High | 0.64 [0.34–1.18] | 0.30 [0.12–0.73] | 0.53 [0.26–1.08] | 0.32 [0.12–0.88] |
| Fast Foods 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 2.14 [0.91–5.02] | 2.41 [0.70–8.23] | 1.62 [0.51–5.10] | 2.86 [0.64–12.64] |
| High | 3.24 [1.21–8.69] | 1.50 [0.43–5.23] | 2.46 [0.79–7.67] | 1.50 [0.37–6.01] |
| Sugar and Sweets 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.04 [0.57–1.90] | 0.86 [0.37–1.92] | 1.03 [0.50–2.14] | 1.01 [0.39–2.59] |
| High | 1.12 [0.61–2.06] | 0.76 [0.33–1.75] | 1.17 [0.57–2.43 | 1.59 [0.65–3.88] |
| Sugar Sweetened Beverages 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.13 [0.67–1.90] | 0.66 [0.34–1.28] | 0.53 [0.22–1.28] | 0.57 [0.22–1.17] |
| High | 1.32 [0.79–2.22] | 0.59 [0.29–1.17] | 0.81 [0.45–1.45] | 0.54 [0.20–1.13] |
| Variables | 12–19 Years | |||
|---|---|---|---|---|
| (n = 498) | ||||
| Overweight 1 | Obesity 1 | Elevated WHtR | Elevated WC | |
| Odds ratio [95%CI] | ||||
| Socio–Demographic Factors | ||||
| Age (years) | 0.99 [0.83–1.18] | 0.91 [0.69–1.20] | 0.99 [089–1.10] | 0.95 [0.77–1.17] |
| Sex | ||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.68 [0.92–3.07] | 5.18 [1.76–15.28] | 1.82 [1.12–2.97] | 0.75 [0.35–1.58] |
| Place of Residence | ||||
| Beirut (Capital) | 1.00 | 1.00 | 1.00 | 1.00 |
| Other Governorates | 0.40 [0.19–0.83] | 0.54 [0.14–2.06] | 1.18 [0.47–2.96] | 1.00 [0.29–3.44] |
| Father’s Education 2 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.53 [0.67–3.50] | 1.26 [0.30–5.22] | 1.70 [0.87–3.31] | 1.14 [0.38–3.42] |
| High | 1.47 [0.63–3.42] | 2.20 [0.56–8.64] | 1.83 [0.90–3.72] | 2.13 [0.75–6.08] |
| Mother’s Education 2 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.84 [0.33–2.09] | 1.33 [0.26–6.86] | 0.67 [0.33–1.36] | 0.48 [0.15–1.55] |
| High | 1.30 [0.54–3.12] | 2.12 [0.50–8.85] | 0.73 [0.36–1.45] | 0.73 [0.25–2.13] |
| Mother’s Working Status | ||||
| Not Working | 1.00 | 1.00 | 1.00 | 1.00 |
| Working | 1.50 [0.69–3.27] | 0.47 [0.10–2.07] | 1.10 [0.60–2.02] | 1.11 [0.43–2.86] |
| Crowding Index | ||||
| <1 person/room | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥1 person/room | 1.74 [0.69–4.40] | 0.96 [0.25–3.65] | 0.74 [0.39–1.41] | 0.73 [0.28–1.95] |
| Parental Obesity | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 3.01 [1.61–5.63] | 2.93 [1.09–7.86] | 2.87 [1.55–5.30] | 1.74 [0.81–3.72] |
| Lifestyle and Dietary Factors | ||||
| Physical Activity 3 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.79 [0.44–1.39] | 0.89 [0.37–2.12] | 0.75 [0.39–1.43] | 0.77 [0.35–1.69] |
| High | 0.62 [0.33–1.05] | 0.43 [0.13–1.33] | 0.53 [0.26–1.09] | 0.53 [0.21–1.31] |
| Sedentary time (h/day) | 1.12 [1.03–1.21] | 1.20 [1.06–1.35] | 1.27 [1.13–1.43] | 1.10 [1.01–1.22] |
| Daily Breakfast Consumption | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.62 [0.28–1.40] | 1.11 [0.28–4.41] | 0.58 [0.24–1.37] | 0.72 [0.27–1.88] |
| Frequency of Eating Out | ||||
| ≤1 time/week | 1.00 | 1.00 | 1.00 | 1.00 |
| >1 time/week | 0.74 [0.47–1.17] | 0.87 [0.42–1.79] | 0.87 [0.52–1.45] | 1.09 [0.60–1.97] |
| Total Daily Energy Intake (Kcal) 2 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.05 [0.56–1.96] | 0.62 [0.23–1.69] | 0.67 [0.33–1.36] | 0.62 [0.28–1.39] |
| High | 0.80 [0.42–1.54] | 0.85 [0.31–2.31] | 0.75 [0.37–1.52] | 0.91 [0.41–2.04] |
| Bread and Cereals 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.18 [0.69–2.02] | 1.71 [0.75–3.92] | 1.21 [0.66–2.23] | 1.21 [0.61–2.39] |
| High | 0.57 [0.32–1.02] | 1.07 [0.43–2.65] | 0.79 [0.41–1.50] | 0.76 [0.36–1.62] |
| Milk and Dairies 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.04 [0.61–1.78] | 0.79 [0.36–1.75] | 1.26 [0.69–2.32] | 1.39 [0.70–2.75] |
| High | 0.56 [0.32–0.98] | 0.50 [0.21–1.20] | 1.17 [0.64–2.15] | 1.04 [0.51–2.12] |
| Meat and equivalent 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.78 [0.44–1.39] | 0.89 [0.35–2.27] | 0.94 [0.49–1.79] | 1.38 [0.64–2.95] |
| High | 1.12 [0.63–1.99] | 1.01 [0.39–2.57] | 1.01 [0.52–1.93] | 1.45 [0.66–3.14] |
| Legumes and Nuts 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.66 [0.39–1.12] | 1.59 [0.76–3.31] | 1.34 [0.76–2.46] | 1.28 [0.64–2.54] |
| High | 0.97 [0.61–1.53] | 0.81 [0.38–1.75] | 1.33 [0.77–2.30] | 1.25 [0.66–2.36] |
| Fruits and Vegetables 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.87 [0.50–1.54] | 0.73 [0.30–1.73] | 0.62 [0.29–1.33] | 0.46 [0.21–1.00] |
| High | 0.69 [0.39–1.21] | 0.87 [0.36–2.06] | 0.61 [0.30–1.24] | 0.60 [0.29–1.21] |
| Added Fats and Oils 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.39 [0.79–2.45] | 0.67 [0.28–1.57] | 0.87 [0.46–1.65] | 0.96 [0.45–2.03] |
| High | 1.19 [0.67–2.13] | 0.92 [0.39–2.18] | 1.31 [0.70–2.45] | 1.63 [0.79–3.35] |
| Fast Foods 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.92 [0.31–2.73] | 0.89 [0.25–1.31] | 1.07 [0.44–2.59] | 1.64 [0.59–4.57] |
| High | 1.40 [0.53–3.66] | 1.34 [0.45–4.03] | 1.46 [0.67–3.16] | 2.05 [0.83–5.08] |
| Sugar and Sweets 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 0.72 [0.41–1.27] | 0.82 [0.35–1.88] | 0.82 [0.44–1.53] | 0.50 [0.25–1.17] |
| High | 0.82 [0.47–1.42] | 0.75 [0.32–1.73] | 0.82 [0.44–1.53] | 0.72 [0.39–1.31] |
| Sugar Sweetened Beverages 4 | ||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 2.49 [1.50–4.12] | 1.74 [0.78–3.88] | 1.77 [1.02–3.07] | 1.62 [0.83–3.13] |
| High | 1.36 [0.80–2.29] | 1.69 [0.76–3.77] | 0.94 [0.52–1.70] | 1.19 [0.60–2.37] |
4. Discussion
5. Conclusions
Acknowledgments
Authors’ Contributions
Conflicts of Interest
References
- Sibai, A.M.; Nasreddine, L.; Mokdad, A.H.; Adra, N.; Tabet, M.; Hwalla, N. Nutrition transition and cardiovascular disease risk factors in the MENA countries: Reviewing the evidence. Ann. Nutr. Metab. 2010, 57, 193–203. [Google Scholar] [CrossRef]
- Wang, Y.; Lobstein, T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 2006, 1, 11–25. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Nasreddine, L.; Ouaijan, K.; Mansour, M.; Adra, N.; Sinno, D.; Hwalla, N. Metabolic syndrome and insulin resistance in obese pre-pubertal children in Lebanon: A primary health concern. Ann. Nutr. Metab. 2010, 57, 135–142. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Pawlak, D.B.; Ludwig, D.S. Childhood obesity: Public-health crisis, common sense cure. Lancet 2002, 360, 473–482. [Google Scholar]
- Krebs, N.F.; Jacobson, M.S.; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics 2003, 112, 424–430. [Google Scholar] [CrossRef]
- Blüher, S.; Molz, E.; Wiegand, S.; Otto, K.P.; Sergeyev, E.; Tuschy, S.; L’Allemand-Jander, D.; Kiess, W.; Holl, R.W.; Adiposity Patients Registry Initiative and German Competence Net Obesity. Body mass index, waist circumference, and waist-to-height ratio as predictors of cardiometabolic risk in childhood obesity depending on pubertal development. J. Clin. Endocrinol. Metab. 2013, 98, 3384–3393. [Google Scholar] [CrossRef]
- Hirschler, V.; Molinari, C.; Maccallini, G.; Aranda, C.; Oestreicher, K. Comparison of different anthropometric indices for identifying dyslipidemia in school children. Clin. Biochem. 2011, 44, 659–664. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Waist circumference and not body mass index explains obesity related health risk. Am. J. Clin. Nutr. 2004, 79, 379–384. [Google Scholar]
- Janssen, I.; Katzmarzyk, P.T.; Srinivasan, S.R.; Chen, W.; Malina, R.M.; Bouchard, C.; Berenson, G.S. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005, 115, 1623–1630. [Google Scholar] [CrossRef]
- Lee, S.; Bacha, F.; Gungor, N.; Arslanian, S.A. Waist circumference is an independent predictor of insulin resistance in black and white youths. J. Pediatr. 2006, 148, 188–194. [Google Scholar]
- Maffeis, C.; Banzato, C.; Talamini, G.; Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Ashwell, M. A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message—“Keep your waist circumference to less than half your height”. Int. J. Obes. 2006, 30, 988–992. [Google Scholar] [CrossRef]
- Freedman, D.S.; Kahn, H.S.; Mei, Z.; Grummer-Strawn, L.M.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: The Bogalusa Heart Study. Am. J. Clin. Nutr. 2007, 86, 33–40. [Google Scholar]
- Mushtaq, M.U.; Gull, S.; Abdullah, H.M.; Shahid, U.; Shad, M.A.; Akram, J. Waist circumference, waist-hip ratio and waist-height ratio percentiles and central obesity among Pakistani children aged five to twelve years. BMC Pediatr. 2011, 11, 105. [Google Scholar] [CrossRef]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef]
- Lebanese Republic Ministry of Social Affairs; Central Administration for Statistics and the United Nations Development Programme (UNDP). Living Conditions of Households—The National Survey of Household Living Conditions; MOSA: Beirut, Lebanon, 2004. [Google Scholar]
- Sibai, A.M.; Hwalla, N.; Adra, N.; Rahal, B. Prevalence and covariates of obesity in Lebanon: Findings from the first epidemiological study. Obes. Res. 2003, 11, 1353–1361. [Google Scholar] [CrossRef]
- Nasreddine, L.; Naja, F.; Chamieh, M.C.; Adra, N.; Sibai, A.M.; Hwalla, N. Trends in overweight and obesity in Lebanon: Evidence from two national cross-sectional surveys (1997 and 2009). BMC Public Health 2012, 12, 798. [Google Scholar] [CrossRef]
- Lee, R.; Nieman, D. Nutritional Assessment, 4th ed.; McGraw-Hill Companies: Boston, NY, USA, 2007. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 2002, 246, 1–190. [Google Scholar]
- Zimmet, P.; Alberti, K.G.; Kaufman, F. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar]
- Fernandez, J.R.; Redden, D.T.; Pietrobelli, A.; Allison, D.B. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J. Pediatr. 2004, 145, 439–444. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Borrud, L.; Perloff, B.; LaComb, R. Improved method for the 24-h dietary recall for use in national surveys. FASEB J. 1999, 13, A603. [Google Scholar]
- Gharib, M.; Rasheed, P. Obesity among Bahrani children and adolescents: Prevalence and associated factors. JBMS 2008, 20, 114–123. [Google Scholar]
- Nasreddine, L.; Sibai, A.M.; Mrayati, M.; Adra, N.; Hwalla, N. Adolescent obesity in Syria: Prevalence and associated factors. Child Care Health Dev. 2010, 36, 404–413. [Google Scholar] [CrossRef]
- Bener, A. Prevalence of obesity, overweight, and underweight in Qatari adolescents. Food Nutr. Bull. 2006, 27, 39–45. [Google Scholar]
- Malik, M.; Bakir, A. Prevalence of overweight and obesity among children in the United Arab Emirates. Obes. Rev. 2006, 8, 15–20. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; Lamb, M.M.; Flegal, K.M. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 2010, 303, 242–249. [Google Scholar] [CrossRef]
- Taheri, F.; Kazemi, T. Prevalence of overweight and obesity in 7 to 18 year-old children in Birjand/Iran. Iran. J. Pediatr. 2009, 19, 135–140. [Google Scholar]
- El Mouzan, M.I.; Foster, P.J.; Herbish, A.S.; Al Salloum, A.A.; Al Omer, A.A.; Qurachi, M.M.; Kecojevic, T. Prevalence of overweight and obesity in Saudi children and adolescents. Ann. Saudi Med. 2010, 30, 203–208. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Neuhauser, H.; Schaffrath Rosario, A.; Schienkiewitz, A. Abdominal obesity in German adolescents defined by waist-to-height ratio and its association to elevated blood pressure: The KiGGS Study. Obes. Facts 2013, 6, 165–175. [Google Scholar] [CrossRef]
- Papalia, T.; Greco, R.; Lofaro, D.; Mollica, A.; Roberti, R.; Bonofiglio, R. Anthropometric measures can better predict high blood pressure in adolescents. J. Nephrol. 2013, 26, 899–905. [Google Scholar] [CrossRef]
- Gröber-Grätz, D.; Widhalm, K.; de Zwaan, M.; Reinehr, T.; Bluher, S.; Schwab, K.O.; Wiegand, S.; Holl, R.W. Body mass index or waist circumference: Which is the better predictor for hypertension and dyslipidemia in overweight/obese children and adolescents? Association of cardiovascular risk related to body mass index or waist circumference. Horm. Res. Paediatr. 2013, 80, 170–178. [Google Scholar] [CrossRef]
- Al-Almaie, S.M. Prevalence of obesity and overweight among Saudi adolescents in Eastern Saudi Arabia. Saudi Med. J. 2006, 26, 607–611. [Google Scholar]
- Hassapidou, M.; Fotiadou, E.; Maglara, E.; Papadopoulou, S.K. Energy intake, diet composition, energy expenditure, and body fatness of adolescents in Northern Greece. Obesity 2006, 14, 855–862. [Google Scholar] [CrossRef]
- Hwalla, N.; Sibai, A.M.; Adra, N. Adolescent Obesity and Physical Activity. World Rev. Nutr. Diet. 2005, 94, 42–50. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Oliveira, A.C.; Almeida, M.S.; Oliveira, N.; Adan, L. Influence of the family nucleus on obesity in children from northeastern Brazil: A cross-sectional study. BMC Public Health 2007, 7, 235. [Google Scholar] [CrossRef]
- Deshmukh-Taskar, P.R.; Radcliffe, J.D.; Liu, Y.; Nicklas, T.A. Do breakfast skipping and breakfast type affect energy intake, nutrient intake, nutrient adequacy, and diet quality in young adults? NHANES 1999–2002. J. Am. Coll. Nutr. 2010, 29, 407–418. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczynski, M. Systematic review demonstrating that breakfast consumption influences body weight outcomes in children and adolescents in Europe. Crit. Rev. Food Sci. Nutr. 2010, 50, 113–119. [Google Scholar] [CrossRef]
- Maddah, M.; Nikooyeh, B. Factors associated with overweight in children in Rasht, Iran: Gender, maternal education, skipping breakfast and parental obesity. Public Health Nutr. 2009, 13, 196–200. [Google Scholar] [CrossRef]
- Lioret, S.; Volatier, J.L.; Lafay, L.; Touvier, M.; Maire, B. Is food portion size a risk factor of childhood overweight? Eur. J. Clin. Nutr. 2009, 63, 382–391. [Google Scholar] [CrossRef]
- Utter, J.; Scragg, R.; Mhurchu, C.N.; Schaaf, D. At-home breakfast consumption among New Zealand children: Associations with body mass index and related nutrition behaviors. J. Am. Diet. Assoc. 2007, 107, 570–576. [Google Scholar] [CrossRef]
- Drummond, S.; Crombie, N.; Kirk, T. A critique of the effects of snacking on body weight status. Eur. J. Clin. Nutr. 1996, 50, 779–783. [Google Scholar]
- Niemeier, H.M.; Raynor, H.A.; Lloyd-Richardson, E.E.; Rogers, M.L.; Wing, R.R. Fast food consumption and breakfast skipping: Predictors of weight gain from adolescence to adulthood in a nationally representative sample. J. Adolesc. Health 2006, 39, 842–849. [Google Scholar] [CrossRef]
- Thompson, O.M.; Ballew, C.; Resnicow, K.; Must, A.; Bandini, L.G.; Cyr, H.; Dietz, W.H. Food purchased away from home as a predictor of change in BMI z-score among girls. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 282–289. [Google Scholar]
- O’Donnell, S.I.; Hoerr, S.L.; Mendoza, J.A.; Tsuei Goh, E. Nutrient quality of fast food kids meals. Am. J. Clin. Nutr. 2008, 88, 1388–1395. [Google Scholar]
- Poti, J.M.; Slining, M.M.; Popkin, B.M. Where are kids getting their empty calories? Stores, schools, and fast-food restaurants each played an important role in empty calorie intake among US children during 2009–2010. J. Acad. Nutr. Diet. 2013. [Google Scholar] [CrossRef]
- Poti, J.M.; Duffey, K.J.; Popkin, B.M. The association of fast food consumption with poor dietary outcomes and obesity among children: Is it the fast food or the remainder of diet? Am. J. Clin. Nutr. 2013. [Google Scholar] [CrossRef]
- Powell, L.M.; Nguyen, B.T. Fast-food and full-service restaurant consumption among children and adolescents: Effect on energy, beverage, and nutrient intake. JAMA Pediatr. 2013, 167, 14–20. [Google Scholar] [CrossRef]
- Paeratakul, S.; Ferdinand, D.P.; Champagne, C.M.; Ryan, D.H.; Bray, G.A. Fast-food consumption among US adults and children: Dietary and nutrient intake profile. J. Am. Diet. Assoc. 2003, 103, 1332–1338. [Google Scholar]
- Anderson, G.H.; Moore, S.E. Dietary proteins in the regulation of food intake and body weight in humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar]
- Barba, G.; Troiano, E.; Russo, P.; Venezia, A.; Siani, A. Inverse association between body mass and frequency of milk consumption in children. Br. J. Nutr. 2005, 93, 15–19. [Google Scholar] [CrossRef]
- Carruth, B.R.; Skinner, J.D. The role of dietary calcium and other nutrients in moderating body fat in preschool children. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 559–566. [Google Scholar] [CrossRef]
- Skinner, J.D.; Bounds, W.; Carruth, B.R.; Ziegler, P. Longitudinal calcium intake is negatively related to children’s body fat indexes. J. Am. Diet. Assoc. 2003, 103, 1626–1631. [Google Scholar] [CrossRef]
- Heaney, R.P.; Davies, K.M.; Barger-Lux, M.J. Calcium and weight: Clinical studies. J. Am. Coll. Nutr. 2002, 21, 152S–155S. [Google Scholar] [CrossRef]
- Zemel, M.B. Nutritional and endocrine modulation of intracellular calcium: Implications in obesity, insulin resistance and hypertension. Mol. Cell. Biochem. 1998, 188, 129–136. [Google Scholar] [CrossRef]
- Haro-Mora, J.J.; García-Escobar, E.; Porras, N.; Alcázar, D.; Gaztambide, J.; Ruíz-Órpez, A.; García-Serrano, S.; Rubio-Martín, E.; García-Fuentes, E.; López-Siguero, J.P.; et al. Children whose diet contained olive oil had a lower likelihood of increasing their body mass index z-score over 1 year. Eur. J. Endocrinol. 2011, 165, 435–439. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Portillo, M.P.; Pico, C.; Macarulla, M.T.; Palou, A. Olive oil feeding up-regulates uncoupling protein genes in rat brown adipose tissue and skeletal muscle. Am. J. Clin. Nutr. 2002, 75, 213–220. [Google Scholar]
- Soriguer, F.; Moreno, F.; Rojo-Martinez, G.; Cardona, F.; Tinahones, F.; Gomez-Zumaquero, J.M.; Garcia Fuentes, E.; Morcillo, S. Redistribution of abdominal fat after a period of food restriction in rats is related to the type of dietary fat. Br. J. Nutr. 2003, 89, 115–122. [Google Scholar] [CrossRef]
- Soriguer, F.; Moreno, F.; Rojo-Martinez, G.; Garcia-Fuentes, E.; Tinahones, F.; Gomez-Zumaquero, J.M.; Cuesta Munoz, A.L.; Cardona, F.; Morcillo, S. Monounsaturated n-9 fatty acids and adipocyte lipolysis in rats. Br. J. Nutr. 2003, 90, 1015–1022. [Google Scholar] [CrossRef]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar]
- De Bourdeaudhuij, I.; Verloigne, M.; Maes, L.; Van Lippevelde, W.; Chinapaw, M.J.; Te Velde, S.J.; Manios, Y.; Androutsos, O.; Kovacs, E.; Dössegger, A.; et al. Associations of physical activity and sedentary time with weight and weight status among 10- to 12-year-old boys and girls in Europe: A cluster analysis within the ENERGY project. Pediatr. Obes. 2013, 8, 367–375. [Google Scholar] [CrossRef]
- Sisson, S.B.; Church, T.S.; Martin, C.K.; Tudor-Locke, C.; Smith, S.R.; Bouchard, C.; Earnest, C.P.; Rankinen, T.; Newton, R.L.; Katzmarzyk, P.T. Profiles of sedentary behavior in children and adolescents: The US national health and nutrition examination survey, 2001–2006. Int. J. Pediatr. Obes. 2009, 4, 353–359. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Gull, S.; Shahid, U.; Shafique, M.M.; Abdullah, H.M.; Shad, M.A.; Siddiqui, A.M. Family-based factors associated with overweight and obesity among Pakistani primary school children. BMC Pediatr. 2011, 11, 114. [Google Scholar] [CrossRef]
- Apfelbacher, C.J.; Loerbroks, A.; Cairns, J.; Behrendt, H.; Ring, J.; Krämer, U. Predictors of overweight and obesity in five to seven-year-old children in Germany: Results from cross-sectional studies. BMC Public Health 2008, 8, 171. [Google Scholar] [CrossRef]
- Hawkins, S.S.; Cole, T.J.; Law, C.; The Millennium Cohort Study Child Health Group. An ecological systems approach to examining risk factors for early childhood overweight: Findings from the UK Millennium Cohort Study. J. Epidemiol. Community Health 2009, 63, 147–155. [Google Scholar]
- Lazzeri, G.; Pammolli, A.; Pilato, V.; Giacchi, M.V. Relationship between 8/9-year-old school children BMI, parents’ BMI and educational level: A cross sectional survey. Nutr. J. 2011, 10, 76. [Google Scholar] [CrossRef]
- Watanabe, E.; Lee, J.S.; Kawakubo, K. Associations of maternal employment and three-generation families with pre-school children’s overweight and obesity in Japan. Int. J. Obes. 2011, 35, 945–952. [Google Scholar] [CrossRef]
- Crawford, P.B.; Obarzanek, E.; Morrison, J.; Sabry, Z.I. Comparative advantage of 3-day food records over 24-h recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J. Am. Diet. Assoc. 1994, 94, 626–630. [Google Scholar] [CrossRef]
- Livingstone, M.B.; Robson, P.J. Measurement of dietary intake in children. Proc. Nutr. Soc. 2000, 59, 279–293. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet. Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- United Nations; Division for Public Administration and Development Management (DPADM); Department of Economic and Social Affairs (DESA). Republic of Lebanon, Public Administration, Country Profile; United Nations: New York, NY, USA, 2004. [Google Scholar]
- Poskitt, E.M. Countries in transition: Underweight to obesity non-stop? Ann. Trop. Paediatr. 2009, 29, 1–11. [Google Scholar] [CrossRef]
- Hoelscher, D.M.; Kirk, S.; Ritchie, L.; Cunningham-Sabo, L.; Academy Positions Committee. Position of the Academy of Nutrition and Dietetics: Interventions for the prevention and treatment of pediatric overweight and obesity. J. Acad. Nutr. Diet. 2013, 113, 1375–1394. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nasreddine, L.; Naja, F.; Akl, C.; Chamieh, M.C.; Karam, S.; Sibai, A.-M.; Hwalla, N. Dietary, Lifestyle and Socio-Economic Correlates of Overweight, Obesity and Central Adiposity in Lebanese Children and Adolescents. Nutrients 2014, 6, 1038-1062. https://doi.org/10.3390/nu6031038
Nasreddine L, Naja F, Akl C, Chamieh MC, Karam S, Sibai A-M, Hwalla N. Dietary, Lifestyle and Socio-Economic Correlates of Overweight, Obesity and Central Adiposity in Lebanese Children and Adolescents. Nutrients. 2014; 6(3):1038-1062. https://doi.org/10.3390/nu6031038
Chicago/Turabian StyleNasreddine, Lara, Farah Naja, Christelle Akl, Marie Claire Chamieh, Sabine Karam, Abla-Mehio Sibai, and Nahla Hwalla. 2014. "Dietary, Lifestyle and Socio-Economic Correlates of Overweight, Obesity and Central Adiposity in Lebanese Children and Adolescents" Nutrients 6, no. 3: 1038-1062. https://doi.org/10.3390/nu6031038