Vitamin A/Retinol and Maintenance of Pluripotency of Stem Cells
Abstract
:1. Introduction
2. Retinol, Retinoic Acid and Cell Differentiation

3. Retinol and Pluripotency of Embryonic Stem (ES) Cells
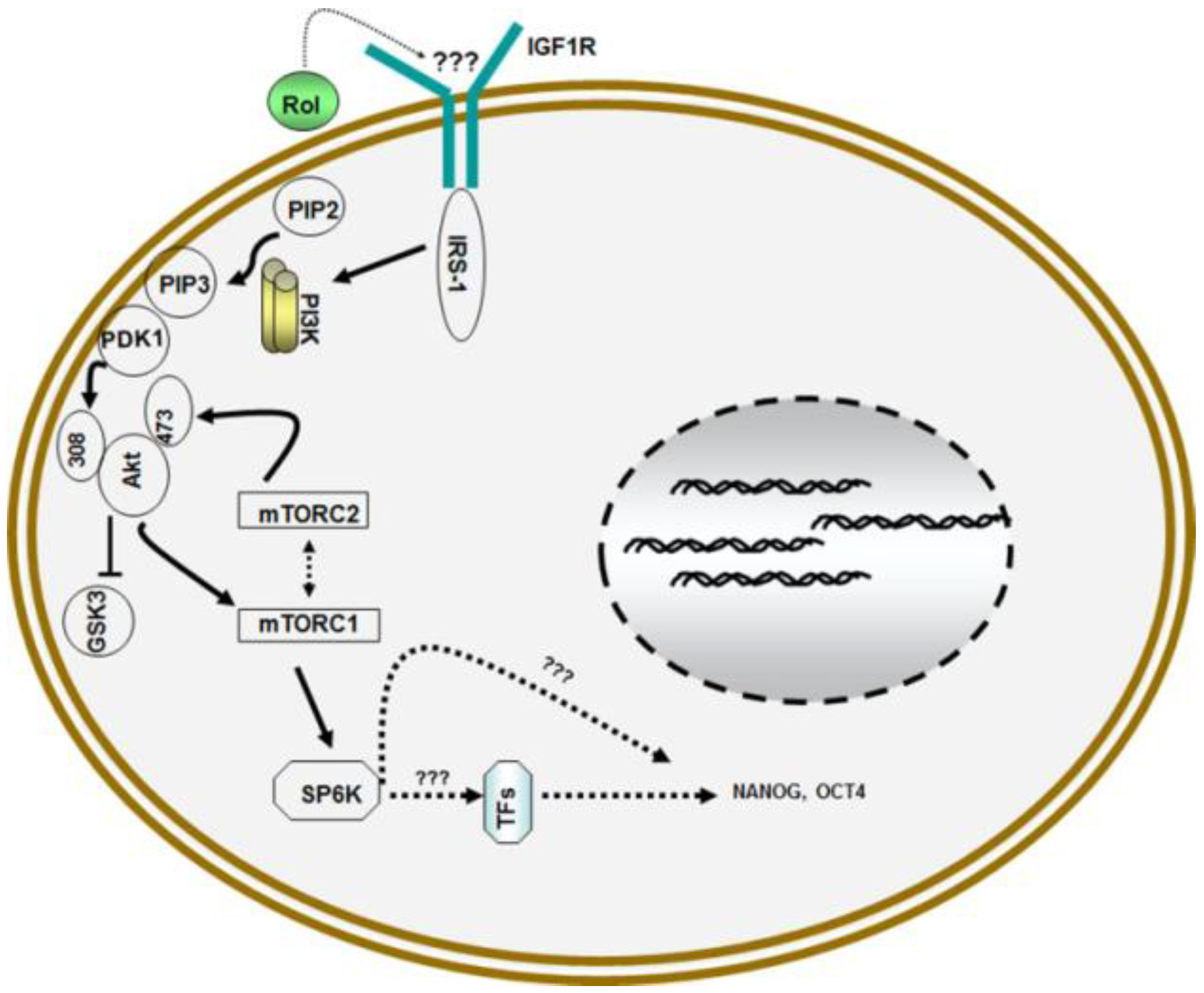
4. Retinol and Proliferation of Germ Line Stem Cells (GSCs)
5. Retinol and Proliferation of Human ESCs
6. Retinol and Amplification of Cancer Stem Cells (CSCs)
7. Stem Cells and Impaired Retinol Metabolism
8. Purity of Stem Cell Population
9. Retinol and Mitochondrial Function
10. Vitamin A and Reproduction
11. Conclusions
Conflicts of Interest
References
- McCollum, E.V.; Davis, M. The necessity of certain lipins in the diet during growth. J. Biol. Chem. 1913, 15, 167–175. [Google Scholar]
- Osborne, T.B.; Mendel, L.B. The relation of growth to the chemical constituents of the diet. J. Biol. Chem. 1913, 145, 311–326. [Google Scholar]
- McCaffery, P.; Dräger, U.C. Regulation of retinoic acid signaling in the embryonic nervous system: A master differentiation factor. Cytokine Growth Factor Rev. 2000, 11, 233–249. [Google Scholar] [CrossRef]
- Zile, M.H. Function of vitamin A in vertebrate development. J. Nutr. 2001, 131, 705–708. [Google Scholar]
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin a in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2000, 22, 347–381. [Google Scholar] [CrossRef]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar] [CrossRef]
- Evans, H.M. The effects of inadequate vitamin a on the sexual physiology of the female. J. Biol. Chem. 1928, 77, 651–654. [Google Scholar]
- Van Berkel, T.J.C. Bringing retinoid metabolism into the 21st century. J. Lipid Res. 2009, 50, 2337–2339. [Google Scholar] [CrossRef]
- Guo, X.; Ruiz, A.; Rando, R.R.; Bok, D.; Gudas, L.J. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: Reduced expression of lecithin: Retinol acyltransferase in carcinoma lines. Carcinogenesis 2000, 21, 1925–1933. [Google Scholar] [CrossRef]
- Napoli, J.L. Retinoic acid biosynthesis and metabolism. FASEB J. 1996, 10, 993–1001. [Google Scholar]
- Wald, G. Molcular basis of visual excitation. Science 1913, 162, 230–239. [Google Scholar]
- Chen, L.; Yang, M.; Dawes, J.; Khillan, J.S. Suppression of ES cell differentiation by retinol (vitamin A) via the over expression of Nanog. Differentiation 2007, 75, 682–693. [Google Scholar] [CrossRef]
- Chen, L; Khillan, J.S. Promotion of feeder-independent self-renewal of embryonic stem cells by retinol (vitamin A). Stem Cells 2008, 26, 1858–1864. [Google Scholar] [CrossRef]
- Chen, L.; Khillan, J.S. A novel signaling by vitamin a/retinol promotes self-renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells 2010, 28, 57–63. [Google Scholar]
- Zhang, S.; Sun, J.; Pan, S.; Zhu, H.; Wang, L.; Hu, Y.; Wang, J.; Wang, F.; Cao, H.; Yan, X.; Hua, J. Retinol (vitamin A) maintains self-renewal of pluripotent germline stem cell (mGSCs) form adult mouse testis. J. Cell Biochem. 2011, 112, 1009–1021. [Google Scholar] [CrossRef]
- Sharma, R.B.; Wang, Q.; Khillan, J.S. Amplification of tumor inducing putative cancer stem cells (CSCs) by vitamin A/retinol form mammary tumors. Biochem. Biophys. Res. Commun. 2013, 36, 625–631. [Google Scholar] [CrossRef]
- Acin-Perez, T.; Hoyos, B.; Zhao, F.; Vinogradov, V.; Fischman, D.A.; Harris, R.A.; Leitges, M.; Wongsiriroj, N.; Blaner, W.S.; Manfredi, G.; et al. Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis. FASEB J. 2010, 24, 627–636. [Google Scholar] [CrossRef]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Chambon, P.A. Decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar]
- Lefebvre, P.; Martin, P.J.; Flajollet, S.; Dedieu, S.; Billaut, X.; Lefebvre, B. Transcriptional activities of retinoic acid receptors. Vitam. Horm. 2005, 70, 199–264. [Google Scholar] [CrossRef]
- Senoo, H.; Kojima, N.; Sato, M. Vitamin A-storing cells (stellate cells). Vitam. Horm. 2007, 75, 131–159. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of digestion and absorption of dietary vitamin A. Annu. Rev. Nutr. 2005, 25, 87–103. [Google Scholar] [CrossRef]
- Goodman, D.S. Plasma Retinol Binding Protein in the Retinoids; Sporn, M.B., Roberts, A.B., Goodman, D.S., Orlando, F.L., Eds.; Academic Press: Salt Lake, UT, USA, 1984; pp. 41–88. [Google Scholar]
- Gudas, L.J. Retinoids and vertebrate development. J. Biol. Chem. 1994, 269, 15399–15402. [Google Scholar]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–923. [Google Scholar] [CrossRef]
- Chen, Y.; Thompson, D.C.; Koppaka, V.; Jester, J.V.; Vasiliou, V. Ocular aldehyde dehydrogenases: Protection against ultraviolet damage and maintenance of transparency for vision. Prog. Retin. Eye Res. 2013, 33, 28–39. [Google Scholar] [CrossRef]
- Boiani, M.; Scholer, H.R. Regulatory networks in embryo-derived pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2005, 6, 872–881. [Google Scholar] [CrossRef]
- Smith, A.G.; Nichols, J.; Robertson, M.; Rathjen, P.D. Differentiation inhibiting activity (DIA/LIF) and mouse development. Dev. Biol. 1992, 151, 339–351. [Google Scholar] [CrossRef]
- Lane, M.A.; Chen, A.C.; Roman, S.D.; Derguini, F.; Gudas, L.J. Removal of LIF (leukemia inhibitory factor) results in increased vitamin A (retinol) metabolism to 4-oxoretinol in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1996, 96, 13524–13529. [Google Scholar]
- Huang, J.; Manning, B.D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 2009, 37, 217–222. [Google Scholar] [CrossRef]
- Wang, R; Liang, J.; Yu, H.-M.; Liang, H.; Shi, Y.-J.; Yang, H.-T. Retinoic acid maintains self-renewal of murine embryonic stem cells via a feedback mechanism. Differentiation 2008, 76, 931–945. [Google Scholar]
- Miyabayashi, T.; Teo, J.L.; Yamamoto, M.; McMillan, M.; Nguyen, C.; Kahn, M. Wnt/β-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc. Natl. Acad. Sci. USA 2007, 104, 5668–5673. [Google Scholar] [CrossRef]
- Ying, Q.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef]
- Chen, L.; Khillan, J.S.; University of Pittsburgh, Pittsburgh, PA, USA. Unpublished work. 2011.
- Wrobel, K.H.; Bickel, D.; Kujat, R. Immunohistochemical study of seminiferous epithelium in adult bovine testis using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen (PCNA). Cell Tissue Res. 1996, 283, 191–201. [Google Scholar] [CrossRef]
- Conrad, S.; Renninger, M.; Hennenlotter, J.; Wiesner, T.; Just, L.; Bonin, M.; Aicher, W.; Bühring, H.J.; Mattheus, U.; Mack, A.; et al. Generation of pluripotent stem cells from adult human testis. Nature 2008, 456, 344–349. [Google Scholar] [CrossRef]
- Guan, K.; Nayernia, K.; Maier, L.S.; Wagner, S.; Dressel, R.; Lee, J.H.; Nolte, J.; Wolf, F.; Li, M.; Engel, W.; et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 2006, 440, 1199–1203. [Google Scholar] [CrossRef]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef]
- Rajala, K.; Vaajasaari, H.; Suuronen, R.; Hovatta, O.; Skottman, H. Effects of the physiochemical culture environment on the stemness and pluripotency of human embryonic stem cells. Stem Cell Stud. 2011, 1. [Google Scholar] [CrossRef]
- Campbell, L.L.; Polyak, K. Breast tumor heterogeneity: Cancer stem cell or clonal evolution. Cell Cycle 2007, 6, 2332–2338. [Google Scholar] [CrossRef]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells current status and evolving complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef]
- Hill, R.P. Identifying cancer stem cells in solid tumors: Case not proven. Cancer Res. 2006, 66, 1891–1896. [Google Scholar] [CrossRef]
- Clarke, M.F.; Fuller, M. Stem cells and cancer: Two faces of eve. Cell 2006, 124, 1111–1115. [Google Scholar] [CrossRef]
- Stingl, J.; Eirew, P.; Ricketson, I.; Shackleton, M.; Vaillant, F.; Choi, D.; Li, H.I.; Eaves, C.J. Purification and unique properties of mammary epithelial stem cells. Nature 2006, 439, 993–997. [Google Scholar]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Huntly, B.J.; Gilliland, D.G. Cancer biology: Summing up cancer stem cells. Nature 2005, 435, 1169–1170. [Google Scholar] [CrossRef]
- Chen, N.; Onisko, B.; Napoli, J.L. The nuclear transcription factor RARα associates with neuronal RNA granules and suppresses translation. J. Biol. Chem. 2008, 283, 20841–20847. [Google Scholar] [CrossRef]
- Aoto, J.; Nam, C.I.; Poon, M.M.; Ting, P.; Chen, L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron 2008, 60, 308–320. [Google Scholar] [CrossRef]
- Thompson, D.A.; Gal, A. Vitamin A metabolism in the retinal pigment epithelium: Genes, mutations, and diseases. Prog. Retin. Eye Res. 2003, 22, 683–703. [Google Scholar] [CrossRef]
- Bhatia, H.; Sharma, R.; Dawes, J.; Khillan, J.S. Maintenance of feeder free anchorage independent cultures of ES and iPS cells by retinol/vitamin A. J. Cell Biochem. 2012, 113, 3002–3010. [Google Scholar] [CrossRef]
- Hoyos, B.; Imam, A.; Chua, R.; Swenson, C.; Tong, G.-X.; Levi, E.; Noy, N.; Hammerling, U. The cysteine-rich regions of the regulatory domains of Raf and protein kinase C as retinoid receptors. J. Exp. Med. 2000, 192, 835–845. [Google Scholar] [CrossRef]
- Imam, A.; Hoyos, B.; Swenson, C.; Chua, R.; Levi, E.; Viriya, E.; Hammerling, U. Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J. 2000, 15, 29–30. [Google Scholar]
- Wolbach, S.B.; Howe, P.R. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef]
- Howell, J.M.; Thompson, J.N.; Pitt, G.A.J. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid, I. The male rat. J. Reprod. Fertil. 1963, 5, 159–167. [Google Scholar] [CrossRef]
- Eskild, W.; Hansson, V. Vitamin A Functions in the Reproductive Organs. In Vitamin A in Health and Disease; Blomhoff, R., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 531–559. [Google Scholar]
- Packer, A.I.; Wolgemuth, D.J. Genetic and Molecular Approaches to Understanding the Role of Retinoids in Mammalian Spermatogenesis. In Retinoids: The Biochemical and Molecular Basis of Vitamin A and Retinoid Action; Nau, H., Blaner, W.S., Eds.; Springer-Verlag: Berlin, Germany, 1999; pp. 347–368. [Google Scholar]
- Dowling, J.E.; Wald, G. The biological function of vitamin A acid. Proc. Natl. Acad. Sci. USA 1960, 46, 587–592. [Google Scholar] [CrossRef]
- Thomson, J.N.; Howell, J.M.; Pitt, G.A. Vitamin A and reproduction in rats. Proc. R. Soc. Lond. 1964, 159, 510–535. [Google Scholar] [CrossRef]
- Chung, S.S.; Wolgemuth, D.J. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet. Genome Res. 2004, 105, 189–202. [Google Scholar] [CrossRef]
- Huang, H.F.; Hembree, W.C. Spermatogenic response to vitamin A in vitamin A deficient rats. Biol. Reprod. 1979, 21, 891–904. [Google Scholar] [CrossRef]
- Van Pelt, A.M.; de Rooij, D.G. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology 1991, 128, 697–704. [Google Scholar] [CrossRef]
- Griswold, M.D.; Bishop, P.D.; Kim, K.H.; Ping, R.; Siiteri, J.E.; Morales, C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N. Y. Acad. Sci. 1989, 564, 154–172. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Liaw, L.; Hogan, B.L. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development 1998, 125, 1103–1112. [Google Scholar]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes. Dev. 1999, 13, 424–436. [Google Scholar] [CrossRef]
- Fujiwara, T.; Dunn, N.R.; Hogan, B.L. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc. Natl. Acad. Sci. USA 2001, 98, 13739–13744. [Google Scholar] [CrossRef]
- Baleato, R.M.; Aitken, R.J.; Roman, S.D. Vitamin A regulation of BMP4 expression in the male germ line. Dev. Biol. 2005, 286, 78–90. [Google Scholar] [CrossRef]
- Alapatt, P.; Guo, F.; Komanetsky, S.M.; Wang, S.; Cai, J.; Sargsyan, A.; Rodríguez Díaz, E.; Bacon, B.T.; Aryal, P.; Graham, T.E. Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J. Biol. Chem. 2013, 288, 1250–1265. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. STRA6-catalyzed vitamin A influx, efflux, and exchange. J. Membr. Biol. 2012, 245, 731–745. [Google Scholar] [CrossRef]
- See, A.W.; Kaiser, M.E.; White, J.C.; Clagett-Dame, M.A. Nutritional model of late embryonic vitamin A deficiency produces defects in organogenesis at a high penetrance and reveals new roles for the vitamin in skeletal development. Dev. Biol. 2008, 316, 171–190. [Google Scholar] [CrossRef]
- Sporn, M.B.; Newton, D.L. Chemoprevention of cancer with retinoids. Fed. Proc. 1979, 38, 2528–2534. [Google Scholar]
- Goodman, G.E.; Alberts, D.S.; Ernest, D.L.; Meyskens, F.L. Phase I trial of retinol in cancer patients. J. Clin. Oncol. 1983, 1, 394–399. [Google Scholar]
- Goodman, G.E.; Alberts, D.S.; Meyskens, F.L. Retinol, vitamins, and cancer prevention: 25 Years of learning and relearning. J. Clin. Oncol. 2008, 26, 5495–5496. [Google Scholar] [CrossRef]
- Lee, I.-M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. B carotene supplementation and incidence of cancer and cardiovascular disease: The Women’s Health Study. J. Natl. Cancer Inst. 1999, 91, 2102–2106. [Google Scholar] [CrossRef]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. Can dietary β-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Chatterjee, N.; Peters, U.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomark. Prev. 2007, 16, 962–968. [Google Scholar] [CrossRef]
- Bertram, J.S.; Kolonel, L.N.; Meyskens, F.L. Rationale and strategies for chemoprevention of cancer in humans. Cancer Res. 1987, 47, 3012–3031. [Google Scholar]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Wong, D.J.; Liu, H.; Ridky, T.W.; Cassarino, D.; Segal, E.; Chang, H.Y. Module map of stem cell genes guides creation epithelial cancer stem cells. Cell Stem Cell 2008, 2, 333–344. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khillan, J.S. Vitamin A/Retinol and Maintenance of Pluripotency of Stem Cells. Nutrients 2014, 6, 1209-1222. https://doi.org/10.3390/nu6031209
Khillan JS. Vitamin A/Retinol and Maintenance of Pluripotency of Stem Cells. Nutrients. 2014; 6(3):1209-1222. https://doi.org/10.3390/nu6031209
Chicago/Turabian StyleKhillan, Jaspal S. 2014. "Vitamin A/Retinol and Maintenance of Pluripotency of Stem Cells" Nutrients 6, no. 3: 1209-1222. https://doi.org/10.3390/nu6031209



