High Dietary Fat Intake during Lactation Promotes the Development of Social Stress-Induced Obesity in the Offspring of Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Diets
2.2. Stress Parameters

2.3. Histological Analysis of White Adipose Tissue
2.4. Biochemical Analyses of Serum
2.5. mRNA Expression Analysis
2.6. Thiobarbituric Acid Active Substance Assay
2.7. Statistical Analysis
3. Results
3.1. Stress Susceptibility

3.2. Growth Parameters
| CC | HC | CH | HH | Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Stress | − | − | − | − | Mother | Stress | Interaction |
| + | + | + | + | ||||
| Body weight and food intake (g) | |||||||
| 3-week | 9.9 ± 1.0 | 10.9 ± 0.9 | 9.9 ± 0.9 | 11.0 ± 0.6 | <0.05 | ns | ns |
| 10.4 ± 0.2 | 11.8 ± 0.3 | 10.7 ± 0.2 | 11.7 ± 0.3 | ||||
| 7-week | 23.3 ± 0.9 | 24.0 ± 0.9 | 23.6 ± 0.6 | 23.5 ± 0.9 | ns | 0.096 | ns |
| 24.0 ± 0.6 | 24.4 ± 0.7 | 23.8 ± 0.3 | 24.7 ± 0.4 | ||||
| 11-week | 27.7 ± 1.1 | 27.6 ± 1.2 | 30.7 ± 0.6 | 30.5 ± 1.1 | ns | <0.01 | ns |
| 27.9 ± 0.8 | 28.8 ± 0.8 | 30.7 ± 1.1 | 35.3 ± 0.8 | ||||
| Food intake (g/day) | |||||||
| Food intake | 3.0 ± 0.2 | 3.2 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 | ns | <0.05 | ns |
| 3.2 ± 0.2 | 3.3 ± 0.2 | 3.1 ± 0.1 | 3.3 ± 0.1 | ||||
| Food intake (g/100 g body weight/day) | |||||||
| Food intake | 0.11 ± 0.00 | 0.12 ± 0.01 | 0.11 ± 0.00 | 0.10 ± 0.00 | <0.01 | ns | ns |
| 0.11 ± 0.00 | 0.11 ± 0.00 | 0.10 ± 0.00 | 0.09 ± 0.00 | ||||
| Calorie intake (calorie/day) | |||||||
| Calorie intake | 10.3 ± 0.0 a | 11.1 ± 0.0 a | 13.5 ± 0.0 d | 12.2 ± 0.0 b, c | <0.01 | 0.059 | <0.01 |
| 11.0 ± 0.4 a | 11.3 ± 0.3 a | 12.9 ± 0.4 c, d | 13.2 ± 0.3 c, d | ||||
| Tissue weight (g/100 g body weight) | |||||||
| Brain | 1.78 ± 0.05 | 1.81 ± 0.07 | 1.56 ± 0.03 | 1.62 ± 0.05 | ns | <0.05 | ns |
| 1.72 ± 0.03 | 1.75 ± 0.04 | 1.57 ± 0.05 | 1.43 ± 0.05 | ||||
| Heart | 0.53 ± 0.04 | 0.53 ± 0.03 | 0.49 ± 0.02 | 0.47 ± 0.02 | ns | ns | ns |
| 0.52 ± 0.03 | 0.52 ± 0.03 | 0.44 ± 0.03 | 0.42 ± 0.02 | ||||
| Kidney | 1.29 ± 0.05 | 1.17 ± 0.03 | 1.08 ± 0.02 | 1.06 ± 0.01 | <0.05 | 0.067 | ns |
| 1.44 ± 0.16 | 1.23 ± 0.02 | 1.18 ± 0.04 | 1.09 ± 0.03 | ||||
| Liver | 4.12 ± 0.11 | 4.15 ± 0.06 | 3.82 ± 0.01 | 3.75 ± 0.02 | ns | <0.01 | ns |
| 4.40 ± 0.08 | 4.25 ± 0.06 | 4.17 ± 0.06 | 4.08 ± 0.17 | ||||
| Lung | 0.73 ± 0.05 | 0.77 ± 0.08 | 0.66 ± 0.03 | 0.72 ± 0.04 | ns | ns | ns |
| 0.69 ± 0.04 | 0.84 ± 0.06 | 0.67 ± 0.06 | 0.66 ± 0.04 | ||||
| Spleen | 0.30 ± 0.02 | 0.30 ± 0.02 | 0.26 ± 0.01 | 0.27 ± 0.01 | ns | ns | ns |
| 0.29 ± 0.02 | 0.26 ± 0.01 | 0.27 ± 0.02 | 0.26 ± 0.03 | ||||
| White adipose tissue | |||||||
| Epididymal | 0.31 ± 0.04 d | 0.24 ± 0.07 d | 0.86 ± 0.09 b, c | 0.88 ± 0.09 b, c | <0.05 | <0.01 | <0.01 |
| 0.47 ± 0.06 d | 0.53 ± 0.06 c, d | 0.93 ± 0.16 b | 1.50 ± 0.07 a | ||||
| Mesenteric | 0.11 ± 0.01 b | 0.08 ± 0.02 e | 0.33 ± 0.04 a, b, c | 0.30 ± 0.03 b, c, d | <0.05 | <0.01 | 0.053 |
| 0.19 ± 0.03 d, e | 0.19 ± 0.03 c, d, e | 0.37 ± 0.05 a, b | 0.510.04 a | ||||
| Perinephric | 0.07 ± 0.01 e | 0.11 ± 0.02 d, e | 0.36 ± 0.04 b | 0.36 ± 0.04 b | <0.01 | <0.01 | <0.05 |
| 0.17 ± 0.04 c, d, e | 0.25 ± 0.04 b, c, d | 0.37 ± 0.09 b, c | 0.67 ± 0.06 a | ||||
3.3. White Adipose Tissue
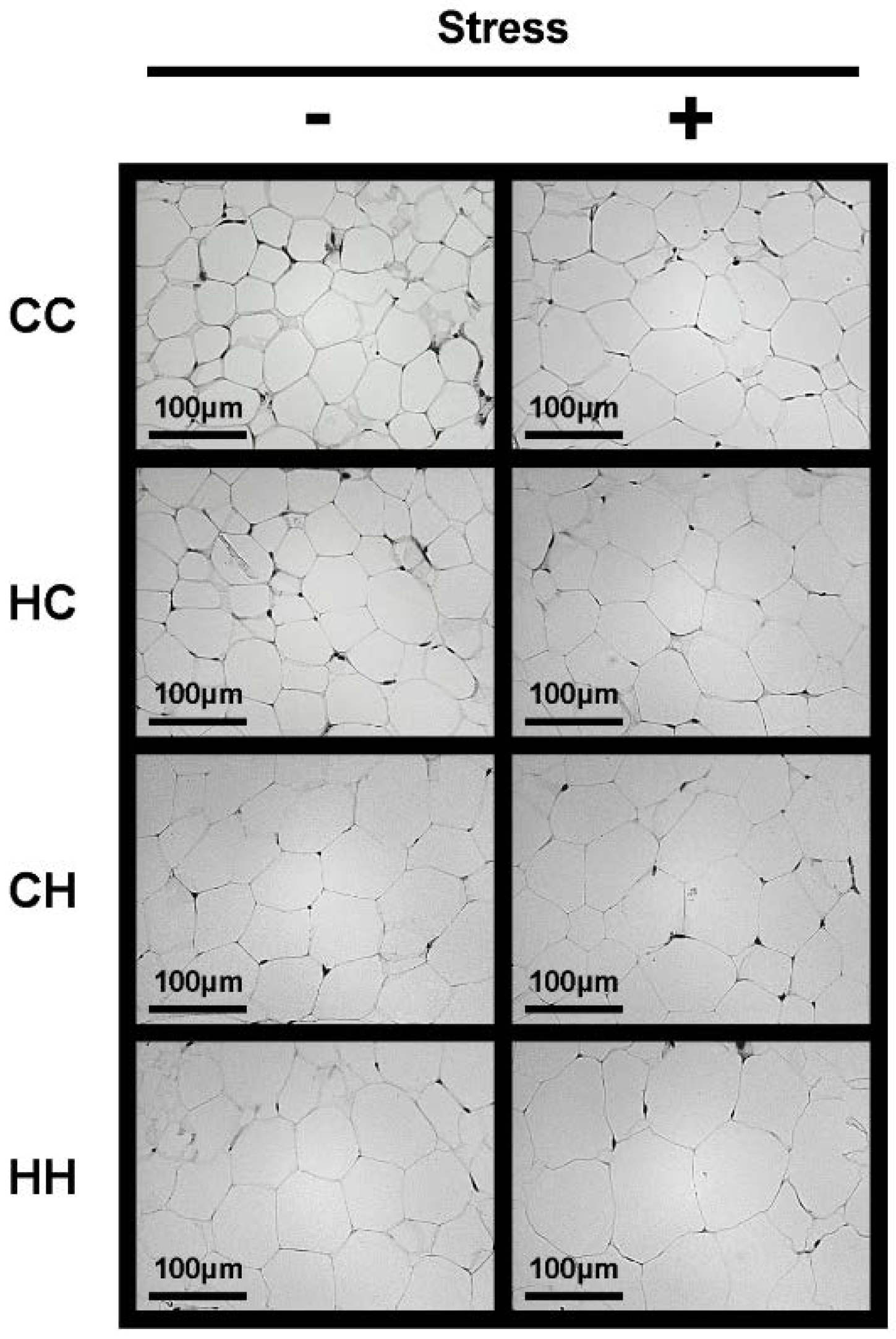

3.4. Expression of mRNA for Genes Related to Lipid Metabolism and Inflammation in White Adipose Tissue
| CC | HC | CH | HH | Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Stress | − | − | − | − | Mother | Stress | Interaction |
| + | + | + | + | ||||
| (Ratio) | |||||||
| Cd36 | 1.00 ± 0.28 | 0.94 ± 0.40 | 1.15 ± 0.43 | 1.89 ± 0.43 | ns | ns | ns |
| 1.37 ± 0.21 | 1.32 ± 0.71 | 0.24 ± 0.03 | 0.81 ± 0.23 | ||||
| Fas | 1.00 ± 0.21 c, d | 0.47 ± 0.13 d | 3.47 ± 1.05 a, b, c | 2.64 ± 0.36 b, c, d | ns | <0.05 | <0.05 |
| 0.57 ± 0.13 c, d | 1.28 ± 0.28 b, c, d | 4.01 ± 1.19 a, b | 5.77 ± 0.63 a | ||||
| Hsl | 1.00 ± 0.44 | 1.09 ± 0.38 | 0.24 ± 0.13 | 0.32 ± 0.12 | ns | < 0.05 | ns |
| 0.59 ± 0.09 | 0.27 ± 0.16 | 0.11 ± 0.01 | 0.09 ± 0.02 | ||||
| Lpl | 1.00 ± 0.14 a, b | 0.38 ± 0.11 b | 1.04 ± 0.13 a, b | 1.39 ± 0.19 a, b | 0.064 | ns | <0.05 |
| 0.51 ± 0.10 a, b | 1.28 ± 0.49 a, b | 0.81 ± 0.06 a, b | 1.43 ± 0.11 a | ||||
| Me | 1.00 ± 0.23 | 0.63 ± 0.11 | 0.92 ± 0.07 | 2.92 ± 0.70 | <0.05 | ns | ns |
| 1.51 ± 0.25 | 1.28 ± 0.47 | 0.78 ± 0.19 | 2.35 ± 0.25 | ||||
| MCP-1 | 1.00 ± 0.23 | 1.28 ± 0.40 | 1.97 ± 0.35 | 3.47 ± 0.83 | <0.01 | <0.05 | ns |
| 1.16 ± 0.12 | 2.00 ± 0.61 | 4.22 ± 0.76 | 4.68 ± 1.09 | ||||
| TNF-α | 1.00 ± 0.43 | 1.34 ± 0.30 | 4.09 ± 1.40 | 4.51 ± 1.77 | <0.01 | ns | ns |
| 2.19 ± 0.57 | 2.33 ± 0.52 | 4.29 ± 0.80 | 6.40 ± 1.39 | ||||
3.5. Lipid and Carbohydrate Parameters in Serum
| CC | HC | CH | HH | Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Stress | − | − | − | − | Mother | Stress | Interaction |
| + | + | + | + | ||||
| TG (mmol/L) | 1.08 ± 0.02 | 0.94 ± 0.07 | 1.44 ± 0.08 | 1.22 ± 0.08 | <0.05 | <0.01 | ns |
| 0.90 ± 0.07 | 0.73 ± 0.08 | 1.09 ± 0.05 | 1.20 ± 0.08 | ||||
| TC (mmol/L) | 1.54 ± 0.09 | 1.70 ± 0.12 | 2.19 ± 0.07 | 2.24 ± 0.03 | ns | ns | ns |
| 1.66 ± 0.04 | 1.61 ± 0.08 | 2.43 ± 0.15 | 2.56 ± 0.17 | ||||
| PL (mmol/L) | 2.27 ± 0.13 d | 2.65 ± 0.07 b, c | 2.69 ± 0.05 a, b, c | 2.95 ± 0.03 a | <0.01 | ns | 0.083 |
| 2.19 ± 0.04 d | 2.00 ± 0.08 c, d | 2.98 ± 0.05 a, b, c | 3.01 ± 0.10 a | ||||
| NEFA (mEq/L) | 0.91 ± 0.06 | 0.91 ± 0.08 | 0.85 ± 0.01 | 0.79 ± 0.02 | <0.01 | ns | |
| 0.76 ± 0.06 | 0.67 ± 0.01 | 0.80 ± 0.03 | 0.77 ± 0.05 | ||||
| Glucose (mmol/L) | 8.4 ± 0.7 | 8.7 ± 0.4 | 9.9 ± 0.6 | 10.8 ± 0.4 | 0.100 | <0.01 | ns |
| 12.6 ± 0.9 | 12.8 ± 0.8 | 14.1 ± 0.8 | 16.4 ± 1.3 | ||||
| Insulin (ng/mL) | 0.43 ± 0.16 | 1.54 ± 0.87 | 0.46 ± 0.11 | 1.56 ± 0.84 | ns | 0.064 | ns |
| 1.88 ± 0.94 | 2.18 ± 1.00 | 2.82 ± 1.14 | 2.03 ± 1.32 | ||||
| Adiponectin (μg/mL) | 3.19 ± 0.62 | 3.89 ± 0.26 | 3.51 ± 0.38 | 3.88 ± 0.27 | ns | <0.05 | ns |
| 2.56 ± 0.12 | 2.86 ± 0.45 | 2.43 ± 0.26 | 3.57 ± 0.59 | ||||
| Leptin (ng/mL) | 0.29 ± 0.01 | 0.34 ± 0.01 | 0.74 ± 0.01 | 0.81 ± 0.04 | <0.01 | <0.01 | ns |
| 0.37 ± 0.02 | 0.47 ± 0.02 | 0.92 ± 0.03 | 1.10 ± 0.13 | ||||
| WAT TBARS (nmol/g WAT) | 2.05 ± 0.19 | 2.82 ± 0.36 | 2.71 ± 0.21 | 2.91 ± 0.15 | ns | <0.05 | ns |
| 3.01 ± 0.29 | 3.02 ± 0.28 | 3.12 ± 0.15 | 3.39 ± 0.41 | ||||
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1191–1209. [Google Scholar] [CrossRef]
- McMillen, I.C.; Rattanatray, L.; Duffield, J.A.; Morrison, J.L.; MacLaughlin, S.M.; Gentili, S.; Muhlhausler, B.S. The early origins of later obesity: Pathways and mechanisms. Adv. Exp. Med. Biol. 2009, 646, 71–81. [Google Scholar] [PubMed]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 61–67. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 10, 1071–1081. [Google Scholar] [CrossRef]
- Khan, I.Y.; Dekou, V.; Douglas, G.; Jensen, R.; Hanson, M.A.; Poston, L.; Taylor, P.D. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Cagampang, F.R.; Mukhtar, D.; Anthony, F.W.; Ohri, S.K.; Hanson, M.A. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 2009, 102, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Howie, G.J.; Sloboda, D.M.; Kamal, T.; Vickers, M.H. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol. 2009, 587, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Tsuduki, T.; Kitano, Y.; Honma, T.; Kijima, R.; Ikeda, I. High dietary fat intake during lactation promotes development of diet-induced obesity in male offspring of mice. J. Nutr. Sci. Vitaminol. 2013, 59, 381–392. [Google Scholar] [CrossRef]
- Moles, A.; Bartolomucci, A.; Garbugino, L.; Conti, R.; Caprioli, A.; Coccurello, R.; Rizzi, R.; Ciani, B.; D’Amato, F.R. Psychosocial stress affects energy balance in mice: Modulation by social status. Psychoneuroendocrinology 2006, 31, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R.; et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007, 13, 801–811. [Google Scholar]
- Sakakibara, H.; Suzuki, A.; Kobayashi, A.; Motoyama, K.; Matsui, A.; Sayama, K.; Kato, A.; Ohashi, N.; Akimoto, M.; Nakayama, T.; Shimoi, K. Social isolation stress induces hepatic hypertrophy in C57BL/6J mice. J. Toxicol. Sci. 2012, 37, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Reus, V.I.; Mellon, S.H. Of sound mind and body: Depression, disease, and accelerated aging. Dialogues Clin. Neurosci. 2011, 13, 21–39. [Google Scholar]
- Toth, M.; Mikics, E.; Tulogdi, A.; Aliczki, M.; Haller, J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm. Behav. 2011, 60, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, M.; Honma, T.; Yanaka, M.; Sato, K.; Shinohara, N.; Tanaka, Y.; Tsuduki, T.; Ikeda, I. Continuous intake of a high-fat diet beyond one generation promotes lipid accumulation in liver and white adipose tissue of female mice. J. Nutr. Biochem. 2012, 23, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Tsuduki, T.; Kikuchi, I.; Kimura, T.; Nakagawa, K.; Miyazawa, T. Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. Food Chem. 2013, 139, 11–23. [Google Scholar] [CrossRef] [PubMed]
- E, S.; Kijima, R.; Honma, T.; Yamamoto, K.; Hatakeyama, Y.; Kitano, Y.; Kimura, T.; Nakagawa, K.; Miyazawa, T.; Tsuduki, T. 1-Deoxynojirimycin attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Exp. Gerontol. 2014, 55, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Palanza, P.; Sacerdote, P.; Panerai, A.E.; Sgoifo, A.; Dantzer, R.; Parmigiani, S. Social factors and individual vulnerability to chronic stress exposure. Neurosci. Biobehav. Rev. 2005, 29, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Finger, B.C.; Dinan, T.G.; Cryan, J.F. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience 2011, 192, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J.R.; Morton, N.M.; Chapman, K.E.; Walker, B.R. Glucocorticoids and 11 β-hydroxysteroid dehydrogenase in adipose tissue. Recent. Prog. Horm. Res. 2004, 59, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Paternain, L.; García-Diaz, D.F.; Milagro, F.I.; González-Muniesa, P.; Martinez, J.A.; Campión, J. Regulation by chronic-mild stress of glucocorticoids, monocyte chemoattractant protein-1 and adiposity in rats fed on a high-fat diet. Physiol. Behav. 2011, 103, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, C.F. Regulation of fatty acid synthase (FAS). Prog. Lipid Res. 1997, 36, 41–53. [Google Scholar] [CrossRef]
- Motoyama, K.; Nakai, Y.; Miyashita, T.; Fukui, Y.; Morita, M.; Sanmiya, K.; Sakakibara, H.; Matsumoto, I.; Abe, K.; Yakabe, T.; et al. Isolation stress for 30 days alters hepatic gene expression profiles, especially with reference to lipid metabolism in mice. Physiol. Genomics 2009, 37, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011, 60, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Wooa, S.J.; Limb, K.; Parkc, S.Y.; Jungc, M.Y.; Limd, H.S.; Jeond, M.-G.; Leed, S.-I.; Parka, B.-H. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids attenuates K/BxN serum-transfer arthritis in fat-1 mice. J. Nutr. Biochem. 2015, 26, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Kano, F.; Shiota, K.; Murata, M. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol. 2009, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Jousse, C.; Parry, L.; Lambert-Langlais, S.; Maurin, A.C.; Averous, J.; Bruhat, A.; Carraro, V.; Tost, J.; Letteron, P.; Chen, P.; et al. Perinatal undernutrition affects the methylation and expression of the leptin gene in adults: Implication for the understanding of metabolic syndrome. FASEB J. 2011, 25, 3271–3278. [Google Scholar] [CrossRef] [PubMed]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91–102. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuduki, T.; Yamamoto, K.; E, S.; Hatakeyama, Y.; Sakamoto, Y. High Dietary Fat Intake during Lactation Promotes the Development of Social Stress-Induced Obesity in the Offspring of Mice. Nutrients 2015, 7, 5916-5932. https://doi.org/10.3390/nu7075257
Tsuduki T, Yamamoto K, E S, Hatakeyama Y, Sakamoto Y. High Dietary Fat Intake during Lactation Promotes the Development of Social Stress-Induced Obesity in the Offspring of Mice. Nutrients. 2015; 7(7):5916-5932. https://doi.org/10.3390/nu7075257
Chicago/Turabian StyleTsuduki, Tsuyoshi, Kazushi Yamamoto, Shuang E, Yu Hatakeyama, and Yu Sakamoto. 2015. "High Dietary Fat Intake during Lactation Promotes the Development of Social Stress-Induced Obesity in the Offspring of Mice" Nutrients 7, no. 7: 5916-5932. https://doi.org/10.3390/nu7075257




