Providing Flaxseed Oil but Not Menhaden Oil Protects against OVX Induced Bone Loss in the Mandible of Sprague-Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
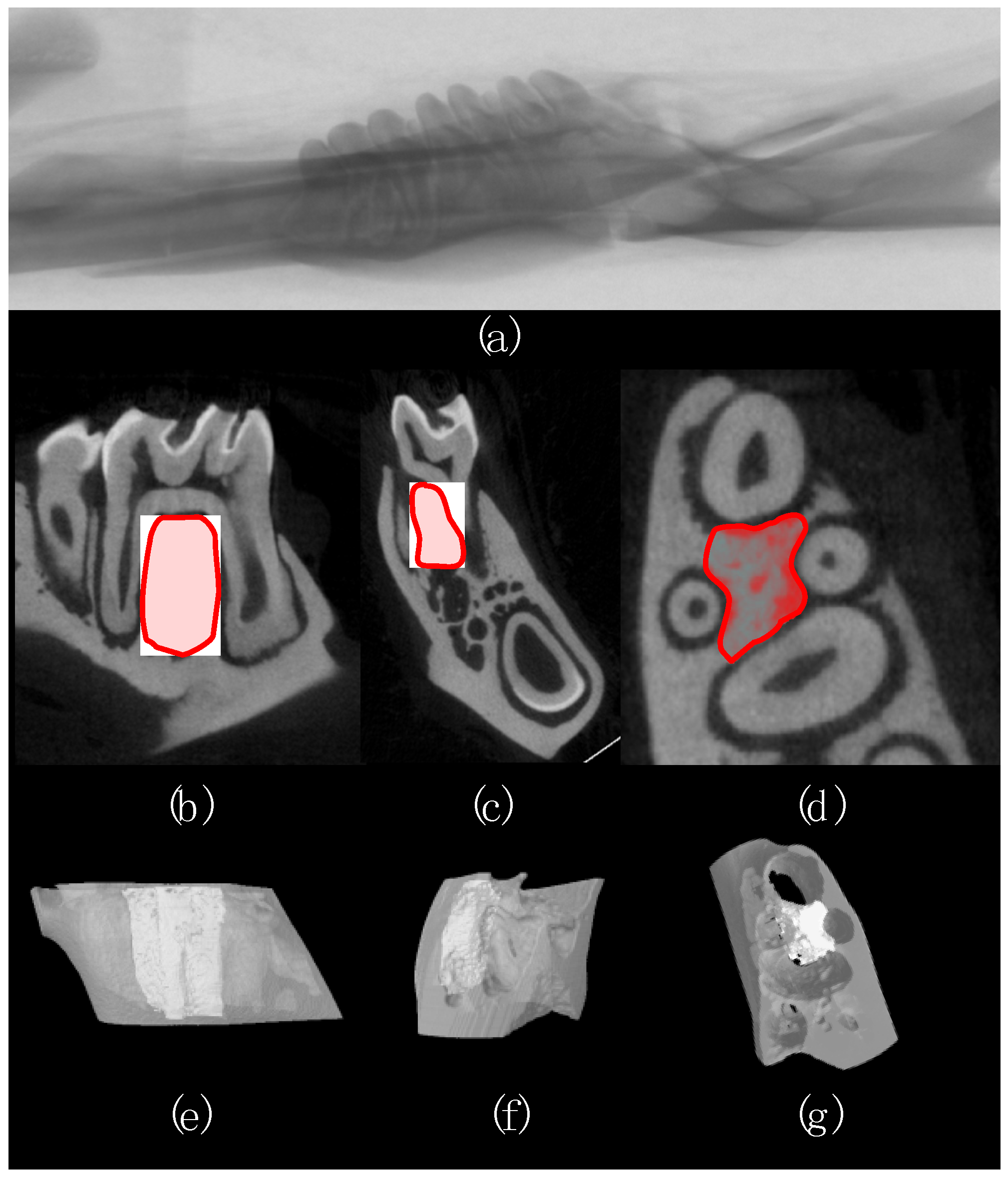
2.2. Micro-Computed Tomography
2.3. Statistical Analyses
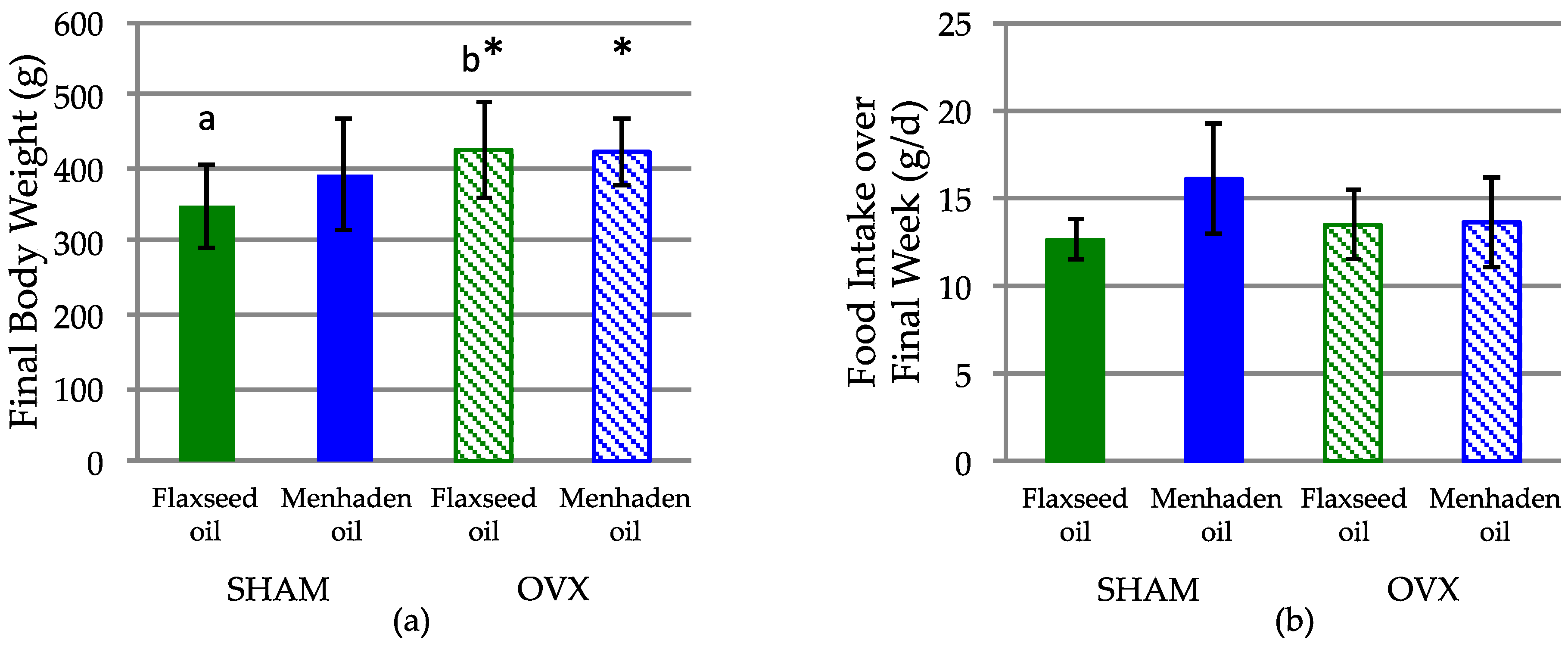
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darcey, J.; Horner, K.; Walsh, T.; Southern, H.; Marjanovic, E.J.; Devlin, H. Tooth loss and osteoporosis: To assess the association between osteoporosis status and tooth number. Br. Dent. J. 2013, 214, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Drozdzowska, B.; Pluskiewicz, W.; Michno, M. Tooth count in elderly women in relation to their skeletal status. Maturitas 2006, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Nas, K.; Kayhan, O.; Atay, M.B.; Akyuz, G.; Sindal, D.; Aksit, R.; Oncel, S.; Dilsen, G.; Cevik, R.; et al. The relation between tooth loss and bone mass in postmenopausal osteoporotic women in Turkey: A multicenter study. J. Bone Miner. Metab. 2003, 21, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Henriques, P.S.; Pinto Neto, A.M. Association between tooth loss and bone mineral density in Brazilian postmenopausal women. J. Clin. Med. Res. 2011, 3, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Kurosu, Y.; Kamiya, T.; Kondo, F.; Yoshinari, N.; Noguchi, T.; Krall, E.A.; Garcia, R.I. Low metacarpal bone density, tooth loss, and periodontal disease in Japanese women. J. Dent. Res. 2001, 80, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Nakamura, K.; Yoshihara, A.; Miyazaki, H. Change in bone mineral density and tooth loss in japanese community-dwelling postmenopausal women: A 5-year cohort study. J. Bone Miner. Metab. 2012, 30, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.M.; Cho, K.H.; Lee, S.H.; Han, S.B.; Han, K.D.; Kim, Y.H. Tooth loss and bone mineral density in postmenopausal south korean women: The 2008–2010 korea national health and nutrition examination survey. Maturitas 2015, 82, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Krall, E.A.; Dawson-Hughes, B.; Papas, A.; Garcia, R.I. Tooth loss and skeletal bone density in healthy postmenopausal women. Osteoporos. Int. 1994, 4, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Krall, E.A.; Garcia, R.I.; Dawson-Hughes, B. Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif. Tissue Int. 1996, 59, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.R.; Hooper, D.A.; Vermilyea, S.G.; Mariotti, A.; Preshaw, P.M. An investigation of the relationship between systemic bone density and clinical periodontal status in post-menopausal asian-american women. Int. Dent. J. 2003, 53, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nicopoulou-Karayianni, K.; Tzoutzoukos, P.; Mitsea, A.; Karayiannis, A.; Tsiklakis, K.; Jacobs, R.; Lindh, C.; van der Stelt, P.; Allen, P.; Graham, J.; et al. Tooth loss and osteoporosis: The osteodent study. J. Clin. Periodontol. 2009, 36, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Suei, Y.; Ohtsuka, M.; Otani, K.; Tanimoto, K.; Hollender, L.G. Relationship between bone mineral density and tooth loss in elderly Japanese women. Dentomaxillofacial Radiol. 1999, 28, 219–223. [Google Scholar] [CrossRef]
- Brennan, D.S.; Singh, K.A.; Liu, P.; Spencer, A. Fruit and vegetable consumption among older adults by tooth loss and socio-economic status. Aust. Dent. J. 2010, 55, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, N.R.; Lin, C.L.; Krall, E. Nutritional status of the older adult is associated with dentition status. J. Am. Diet. Assoc. 2003, 103, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A.; Steele, J. Does the condition of the mouth and teeth affect the ability to eat certain foods, nutrient and dietary intake and nutritional status amongst older people? Public Health Nutr. 2001, 4, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Watanabe, R.; Nishimuta, M.; Hanada, N.; Miyazaki, H. The relationship between dietary intake and the number of teeth in elderly Japanese subjects. Gerodontology 2005, 22, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hollis, J.H. Tooth loss and its association with dietary intake and diet quality in american adults. J. Dent. 2014, 42, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Ward, W.E. The ovariectomized rat as a model for studying alveolar bone loss in postmenopausal women. BioMed Res. Int. 2015, 2015, 635023. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Lee, I.M.; Cole, S.R.; Hennekens, C.H.; Willett, W.C.; Buring, J.E. Fruit and vegetable intake and risk of cardiovascular disease: The women’s health study. Am. J. Clin. Nutr. 2000, 72, 922–928. [Google Scholar] [PubMed]
- Genkinger, J.M.; Platz, E.A.; Hoffman, S.C.; Comstock, G.W.; Helzlsouer, K.J. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in washington county, maryland. Am. J. Epidemiol. 2004, 160, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaelsson, K.; Pettersson-Kymmer, U.; Eriksson, S.; Grodstein, F.; Wolk, A.; Bellavia, A.; Ahmed, L.A.I.; et al. Fruit and vegetable intake and hip fracture incidence in older men and women: The CHANCES project. J. Bone Miner. Res. 2016, 31, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mozaffarian, D.; Willett, W.C.; Feskanich, D. Dietary intake of polyunsaturated fatty acids and risk of hip fracture in men and women. Osteoporos. Int. 2012, 23, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Cauley, J.A.; Frank, G.C.; Neuhouser, M.L.; Robinson, J.G.; Snetselaar, L.; Tylavsky, F.; Wactawski-Wende, J.; Young, A.M.; Lu, B.; et al. Fatty acid consumption and risk of fracture in the women’s health initiative. Am. J. Clin. Nutr. 2010, 92, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, R.; Tuppurainen, M.; Erkkila, A.T.; Penttinen, P.; Karkkainen, M.; Salovaara, K.; Jurvelin, J.S.; Kroger, H. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur. J. Clin. Nutr. 2012, 66, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.; Kerstetter, J.; Kenny, A.; Insogna, K.; Walsh, S.J. An investigation of the association between omega 3 FA and bone mineral density among older adults: Results from the national health and nutrition examination survey years 2005–2008. Osteoporos. Int. 2014, 25, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.H.; Kleppinger, A.; Kenny, A.M. Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J. Am. Geriatr. Soc. 2009, 57, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Nawata, K.; Yamauchi, M.; Takaoka, S.; Yamaguchi, T.; Sugimoto, T. Association of polyunsaturated fatty acid intake with bone mineral density in postmenopausal women. Calcif. Tissue Int. 2013, 93, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.B.; Ward, W.E. PUFAs, bone mineral density, and fragility fracture: Findings from human studies. Adv. Nutr. 2016, 7, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher intakes of fruits and vegetables, beta-carotene, vitamin C, alpha-tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Itomura, M.; Sawazaki, S.; Hamazaki, T. Fish oil reduces tooth loss mainly through its anti-inflammatory effects? Med. Hypotheses 2006, 67, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Taylor, G.W.; Moynihan, P.; Yoshihara, A.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Dietary ratio of n-6 to polyunsaturated fatty acids and periodontal disease in community-based older Japanese: A 3-year follow-up study. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Yoshihara, A.; Moynihan, P.; Watanabe, R.; Taylor, G.W.; Miyazaki, H. Longitudinal relationship between dietary omega-3 fatty acids and periodontal disease. Nutrition 2010, 26, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.Z.; Buettner, C.; Phillips, R.S.; Davis, R.B.; Mukamal, K.J. n-3 fatty acids and periodontitis in us adults. J. Am. Diet. Assoc. 2010, 110, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, E.D.; Kushner, L.J.; Kramer, N.; Kazandjian, G. Pilot study of dietary fatty acid supplementation in the treatment of adult periodontitis. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 213–218. [Google Scholar] [CrossRef]
- Campan, P.; Planchand, P.O.; Duran, D. Pilot study on polyunsaturated fatty acids in the treatment of human experimental gingivitis. J. Clin. Periodontol. 1997, 24, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.A.; Wild, R.D.; Hammond, L.J.; Khalil, D.A.; Juma, S.; Daggy, B.P.; Stoecker, B.J.; Arjmandi, B.H. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Dodin, S.; Lemay, A.; Jacques, H.; Legare, F.; Forest, J.C.; Masse, B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: A randomized, double-blind, wheat germ placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2005, 90, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Ward, W.E.; Lewis, J.E.; Hilditch, J.; Nickell, L.; Wong, E.; Thompson, L.U. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am. J. Clin. Nutr. 2004, 79, 318–325. [Google Scholar] [PubMed]
- Vanpapendorp, D.H.; Coetzer, H.; Kruger, M.C. Biochemical profile of osteoporotic patients on essential fatty-acid supplementation. Nutr. Res. 1995, 15, 325–334. [Google Scholar] [CrossRef]
- Kruger, M.C.; Coetzer, H.; de Winter, R.; Gericke, G.; van Papendorp, D.H. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milano) 1998, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Fraser, W.D.; Rogers, P.J.; Ness, A.R.; Tobias, J.H. Supplementation with a low-moderate dose of long-chain pufa has no short-term effect on bone resorption in human adults. Br. J. Nutr. 2011, 105, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Bassey, E.J.; Littlewood, J.J.; Rothwell, M.C.; Pye, D.W. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre- and postmenopausal women: Two randomized controlled trials of efacal v. Calcium alone. Br. J. Nutr. 2000, 83, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Thompson, D.D.; Simmons, H.A.; Pirie, C.M.; Ke, H.Z. FDA guidelines and animal models for osteoporosis. Bone 1995, 17, 125S–133S. [Google Scholar] [CrossRef]
- Dodd, D.Z.; Rowe, D.J. The relationship between postmenopausal osteoporosis and periodontal disease. J. Dent. Hyg. 2013, 87, 336–344. [Google Scholar] [PubMed]
- Irie, K.; Sakakura, Y.; Tsuruga, E.; Hosokawa, Y.; Yajima, T. Three-dimensional changes of the mandible and alveolar bone in the ovariectomized rat examined by micro-focus computed tomography. J. Jpn. Soc. Periodontol. 2004, 46, 288–293. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, C.L.; Lu, W.W.; Cai, W.X.; Zheng, L.W. Skeletal site-specific response to ovariectomy in a rat model: Change in bone density and microarchitecture. Clin. Oral Implant. Res. 2015, 26, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, C.; Kang, C.; Zhang, B.; Li, Y. Distributional variations in trabecular architecture of the mandibular bone: An in vivo micro-ct analysis in rats. PLoS ONE 2015, 10, e0116194. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Kiliaridis, S.; Rizzoli, R.; Ammann, P. Normal masticatory function partially protects the rat mandibular bone from estrogen-deficiency induced osteoporosis. J. Biomech. 2014, 47, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Rizzoli, R.; Ammann, P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. J. Bone Miner. Res. 2007, 22, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toyooka, E.; Kohno, S.; Ozawa, H.; Ejiri, S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pham, S.M.; Crabbe, D.L. Effects of oestrogen deficiency on rat mandibular and tibial microarchitecture. Dentomaxillofacial Radiol. 2003, 32, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.Y.; Cohen, D.J.; Ward, W.E.; Ma, D.W. Investigating the role of polyunsaturated fatty acids in bone development using animal models. Molecules 2013, 18, 14203–14227. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Fushimi, H.; Inoue, T.; Matsuyama, Y.; Kameyama, M.; Minami, T.; Okazaki, Y.; Noguchi, Y.; Kasama, T. Effect of eicosapentaenoic acid and docosahexaenoic acid in diabetic osteopenia. Diabetes Res. Clin. Pract. 1995, 30, 37–42. [Google Scholar] [CrossRef]
- Li, Y.; Seifert, M.F.; Lim, S.Y.; Salem, N., Jr.; Watkins, B.A. Bone mineral content is positively correlated to n-3 fatty acids in the femur of growing rats. Br. J. Nutr. 2010, 104, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Firth, E.C.; Rogers, C.W.; Moughan, P.J.; Kruger, M.C. Specific effects of gamma-linolenic, eicosapentaenoic, and docosahexaenoic ethyl esters on bone post-ovariectomy in rats. Calcif. Tissue Int. 2007, 81, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Schollum, L.M. Is docosahexaenoic acid more effective than eicosapentaenoic acid for increasing calcium bioavailability? Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.; Gigliotti, J.C.; Smith, B.J.; Altman, S.; Tou, J.C. Consumption of different sources of omega-3 polyunsaturated fatty acids by growing female rats affects long bone mass and microarchitecture. Bone 2011, 49, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Kruger, M.C. Detrimental effect of eicosapentaenoic acid supplementation on bone following ovariectomy in rats. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ejiri, S.; Toyooka, E.; Kohno, S.; Ozawa, H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J. Periodontal Res. 2002, 37, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Li, Y.; Seifert, M.F. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: Actions on bone mineral and serum biomarkers in ovariectomized rats. J. Nutr. Biochem. 2006, 17, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.M.; Jiang, J.M.; Reza-Lopez, S.; Ma, D.W.; Thompson, L.U.; Ward, W.E. Flaxseed combined with low-dose estrogen therapy preserves bone tissue in ovariectomized rats. Menopause 2009, 16, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.M.; Jiang, J.M.; Thompson, L.U.; Ward, W.E. Flaxseed does not enhance the estrogenic effect of low-dose estrogen therapy on markers of uterine health in ovariectomized rats. J. Med. Food 2012, 15, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.B.; Sacco, S.M.; Salmon, P.L.; Ward, W.E. Longitudinal use of micro-computed tomography does not alter microarchitecture of the proximal tibia in sham or ovariectomized sprague-dawley rats. Calcif. Tissue Int. 2016, 98, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Mikkila, V.; Rasanen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent dietary patterns identified from childhood to adulthood: The cardiovascular risk in young finns study. Br. J. Nutr. 2005, 93, 923–931. [Google Scholar] [CrossRef] [PubMed]
| Component (g/kg) | Flaxseed Oil | Menhaden Oil |
|---|---|---|
| Casein + l-Cysteine | 203 | 203 |
| Corn Starch | 357 | 357 |
| Maltodextrin | 132 | 132 |
| Sucrose | 100 | 100 |
| Cellulose | 50 | 50 |
| Mineral Mix 1 | 35 | 35 |
| Vitamin Mix 2 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 |
| TBHQ | 0.022 | 0.022 |
| Safflower Oil | 45 | 70 |
| Cottonseed Oil | 43 | 4 |
| Flaxseed Oil | 22 | - |
| Menhaden Oil | - | 36 |
| SFA | 18.25 | 17.47 |
| MUFA | 18.16 | 18.07 |
| PUFA | 73.55 | 72.42 |
| n-6 | 61.32 | 58.21 |
| n-3 | 12.23 | 11.71 |
| n-6:n-3 | 5.01 | 4.97 |
| Microarchitecture Measurement | Unit | Explanation |
|---|---|---|
| Percent bone volume (BV/TV) | % | Percentage of area within ROI occupied by bone |
| Greater BV/TV is considered positive for bone health | ||
| Connectivity density (Conn. D) | mm−3 | Number of redundant connections between trabecular structures per unit volume |
| Greater Conn. D is considered positive for bone health | ||
| Degree of anisotropy (DA) | No unit | Orientation of the trabecular network, 1-isotropic (lacking orientation), >1 = anisotropic (highly orientated) |
| Greater DA is considered positive for bone health | ||
| Trabecular number (Tb. N.) | mm−1 | Average number of trabeculae per unit length |
| Greater Tb. N. is considered positive for bone health | ||
| Trabecular separation (Tb. Sp.) | mm | Average distance between trabeculae |
| Greater Tb. Sp. is considered negative for bone health | ||
| Trabecular thickness (Tb. Th.) | mm | Average thickness of trabeculae |
| Greater Tb. Th. is considered positive for bone health |
| Trabecular Outcome | SHAM | OVX | p Value | ||||
|---|---|---|---|---|---|---|---|
| Flaxseed Oil | Menhaden Oil | Flaxseed Oil | Menhaden Oil | Interaction | Hormone Status | Diet | |
| BV/TV (%) | 55.87 (5.83) | 59.09 (4.82) | 53.35 (7.61) | 52.22 (5.29) * | NS | 0.05 | NS |
| Conn. D (mm−3) | 915.66 (445.40) | 1420.86 (858.96) | 545.43 (446.03) | 541.75 (230.40) ** | NS | 0.001 | NS |
| DA | 1.32 (0.06) | 1.33 (0.06) | 1.38 (0.08) | 1.33 (0.06) | NS | NS | NS |
| Tb. N. (mm−1) | 11.709 (1.523) | 12.847 (1.463) | 11.011 (1.923) | 10.764 (1.363) * | NS | 0.05 | NS |
| Tb. Sp. (mm) | 0.173 (0.056) | 0.137 (0.056) | 0.203 (0.082) | 0.221 (0.083) * | NS | 0.05 | NS |
| Tb. Th. (mm) | 0.048 (0.001) | 0.046 (0.002) | 0.049 (0.002) | 0.049 (0.001) ** | NS | 0.001 | NS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, A.B.; Ward, W.E. Providing Flaxseed Oil but Not Menhaden Oil Protects against OVX Induced Bone Loss in the Mandible of Sprague-Dawley Rats. Nutrients 2016, 8, 597. https://doi.org/10.3390/nu8100597
Longo AB, Ward WE. Providing Flaxseed Oil but Not Menhaden Oil Protects against OVX Induced Bone Loss in the Mandible of Sprague-Dawley Rats. Nutrients. 2016; 8(10):597. https://doi.org/10.3390/nu8100597
Chicago/Turabian StyleLongo, Amanda B., and Wendy E. Ward. 2016. "Providing Flaxseed Oil but Not Menhaden Oil Protects against OVX Induced Bone Loss in the Mandible of Sprague-Dawley Rats" Nutrients 8, no. 10: 597. https://doi.org/10.3390/nu8100597





