Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and β-Carotene Administration
2.2. Serum Lipoprotein Isolation
2.3. Cholesterol and Triglyceride Measurements in the Lipoprotein Fractions
2.4. HPLC Analysis of Retinol, Retinyl Ester and β-Carotene
2.5. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qPCR)
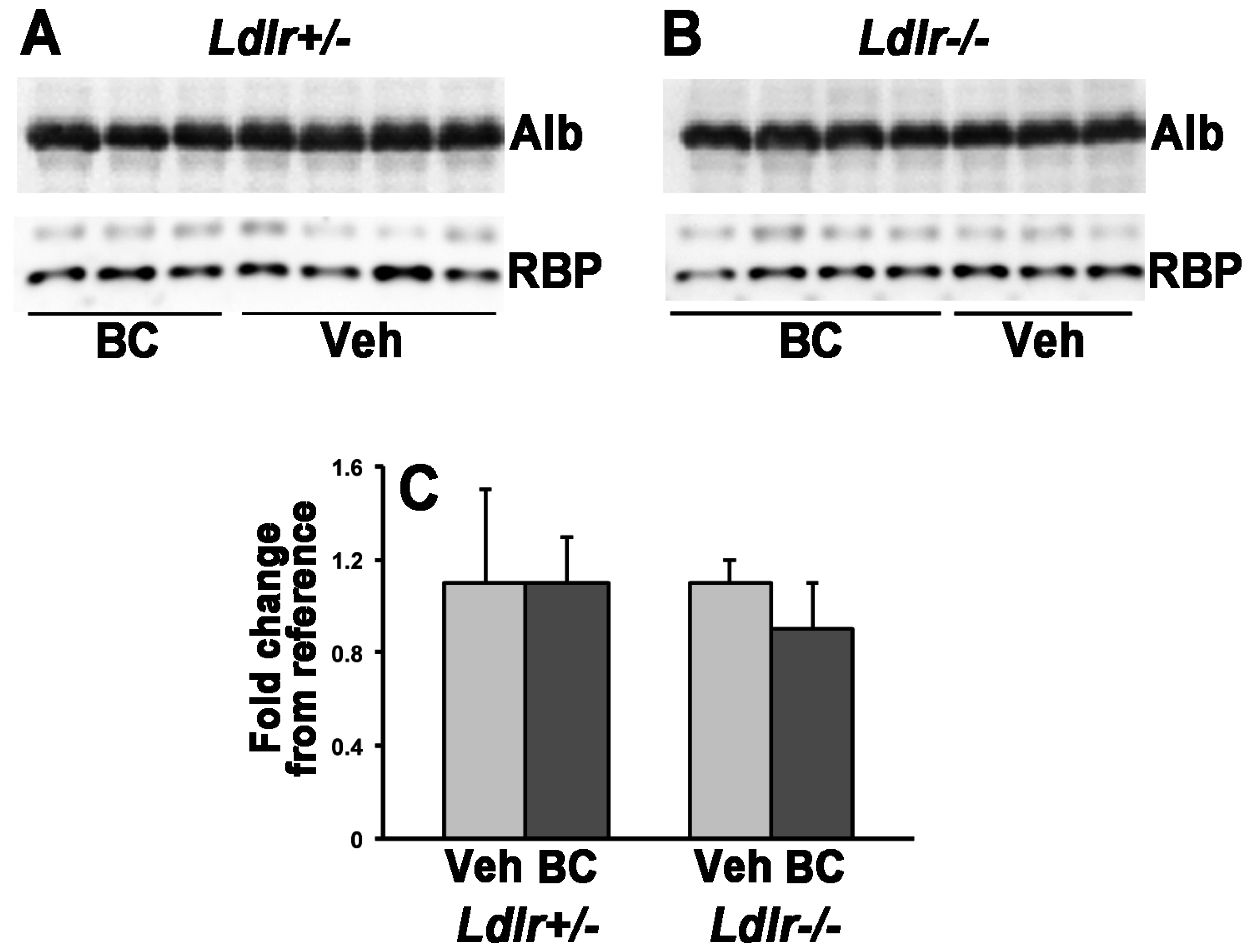
2.6. Western Blot Analysis
2.7. Statistical Analyses
3. Results
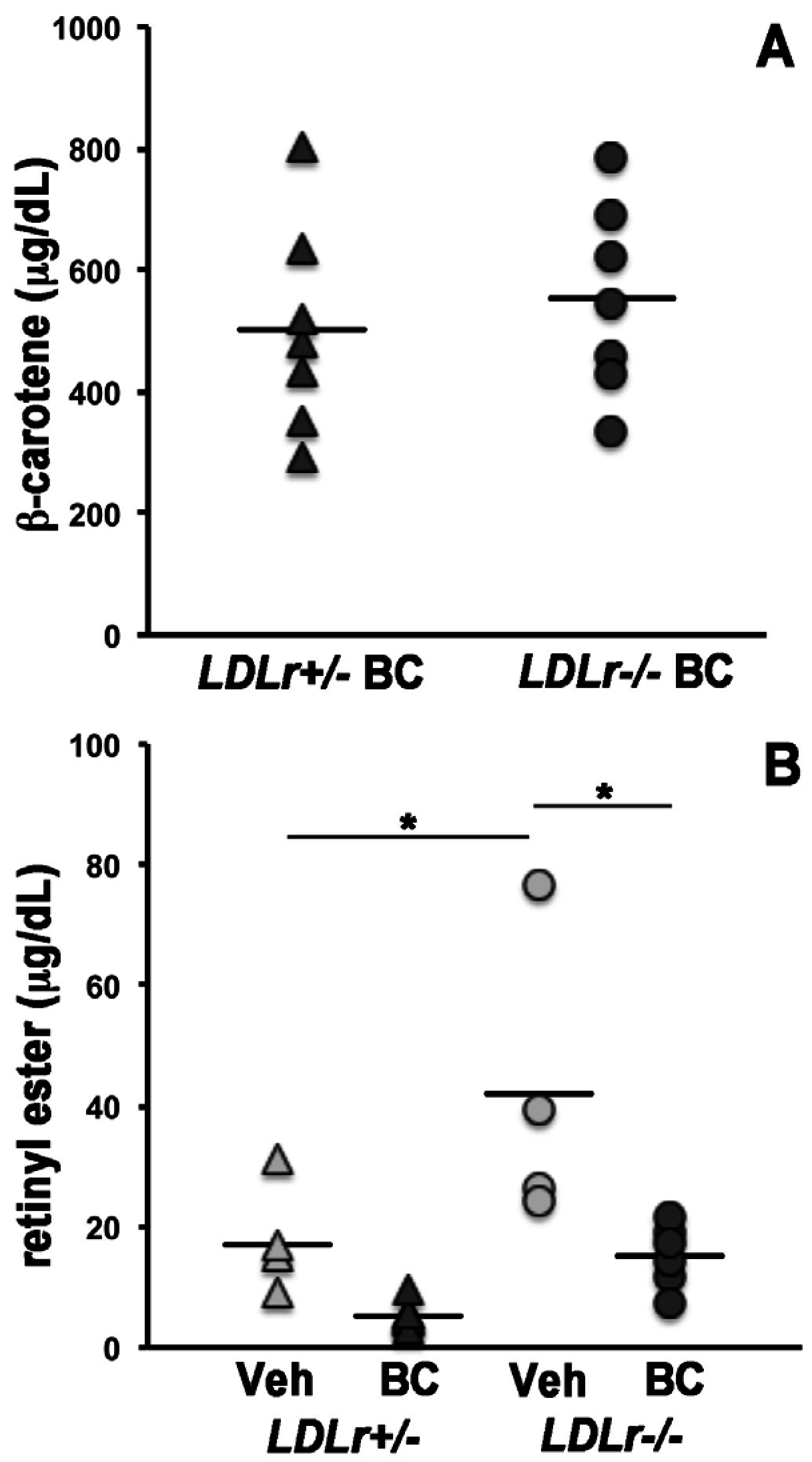
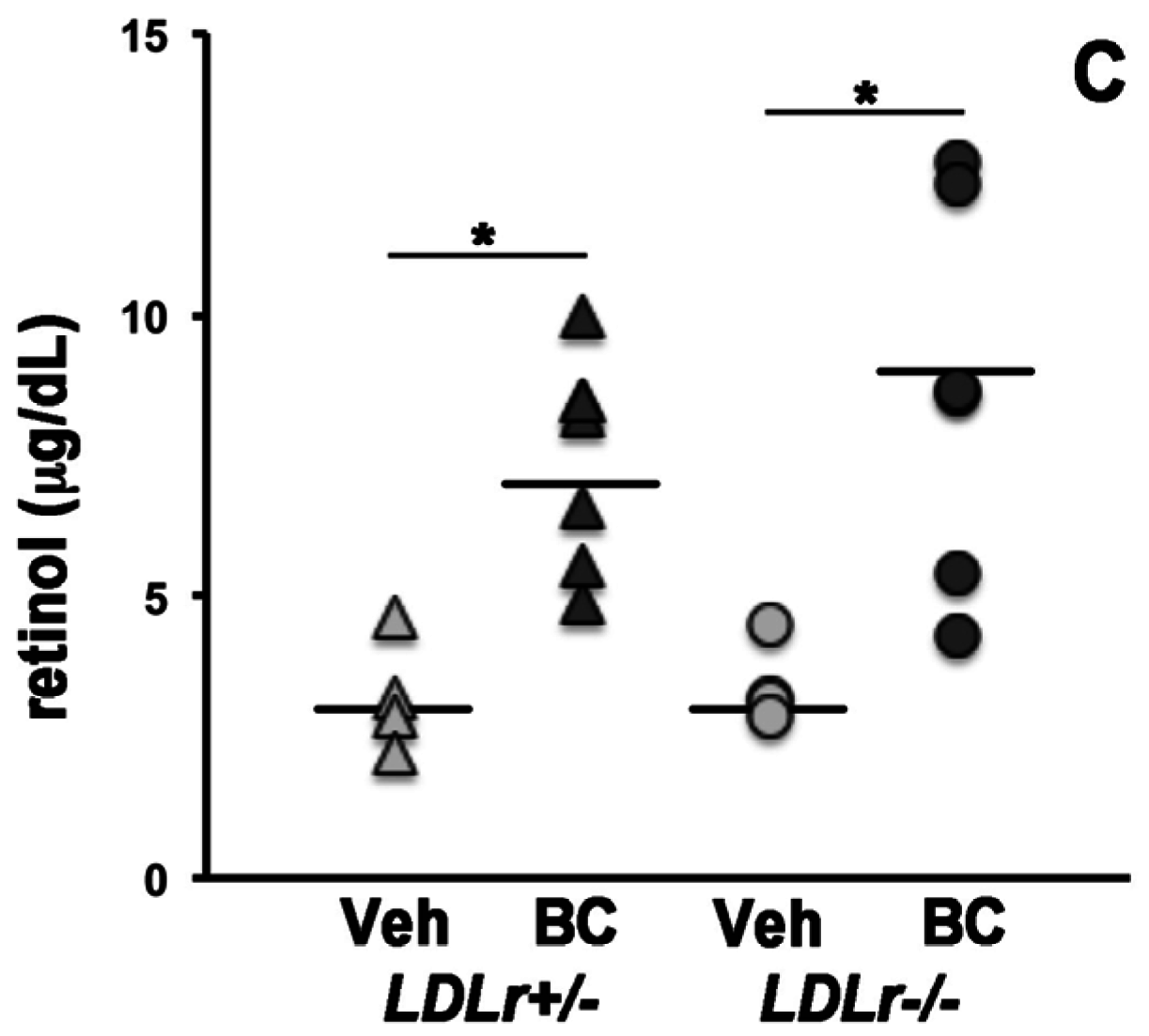
3.1. β-Carotene and Retinoid Concentrations in Maternal Serum and Lipoprotein Particles
3.2. Lack of LDLr Affects β-Carotene Accumulation in Maternal Liver
3.3. Lack of LDLr Does Not Impact β-Carotene Uptake by the Developing Tissues
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef] [PubMed]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef] [PubMed]
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and retinoid signaling: Genomic and nongenomic effects. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Von Lintig, J. Colors with functions: Elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 2010, 30, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Widjaja-Adhi, M.A.; Rodriguez-Santiago, S.; Hessel, S.; Golczak, M.; Palczewski, K.; von Lintig, J. Two carotenoid oxygenases contribute to mammalian provitamin a metabolism. J. Biol. Chem. 2013, 288, 34081–34096. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Wassef, L.; Chung, S.; Jiang, H.; Wyss, A.; Blaner, W.S.; Quadro, L. β-carotene and its cleavage enzyme β-carotene-15,15′-oxygenase (cmoi) affect retinoid metabolism in developing tissues. FASEB J. 2011, 25, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Dela Sena, C.; Narayanasamy, S.; Riedl, K.M.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant human beta-carotene 15,15′-oxygenase (BCO1). J. Biol. Chem. 2013, 288, 37094–37103. [Google Scholar] [CrossRef] [PubMed]
- Wassef, L.; Shete, V.; Costabile, B.; Rodas, R.; Quadro, L. High preformed vitamin a intake during pregnancy prevents embryonic accumulation of intact β-carotene from the maternal circulation in mice. J. Nutr. 2015, 145, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [PubMed]
- Erdman, J.W., Jr.; Bierer, T.L.; Gugger, E.T. Absorption and transport of carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014, 52, 1695–1727. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, L.; Lorent, K.; Torrekens, S.; Van Leuven, F.; Van den Berghe, H. Expression of mouse alpha-macroglobulins, lipoprotein receptor-related protein, LDL receptor, apolipoprotein E, and lipoprotein lipase in pregnancy. J. Lipid Res. 1995, 36, 1774–1786. [Google Scholar] [PubMed]
- Ethier-Chiasson, M.; Duchense, A.; Forest, J.C.; Giguere, Y.; Masse, A.; Mounier, C.; Lafond, J. Influence of maternal lipid profile on placental protein expression of LDLr and SR-BI. Biochem. Biophys. Res. Commun. 2007, 359, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wassef, L.; Quadro, L. Uptake of dietary retinoids at the maternal-fetal barrier: In vivo evidence for the role of lipoprotein lipase and alternative pathways. J. Biol. Chem. 2011, 286, 32198–32207. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L.A. Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32 (Suppl. 2), S218–S221. [Google Scholar] [CrossRef] [PubMed]
- Wassef, L.; Shete, V.; Hong, A.; Spiegler, E.; Quadro, L. Beta-carotene supplementation decreases placental transcription of LDL receptor-related protein 1 in wild-type mice and stimulates placental beta-carotene uptake in marginally vitamin A-deficient mice. J. Nutr. 2012, 142, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 7th ed.; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Costabile, B.K.; Kim, Y.K.; Iqbal, J.; Zuccaro, M.V.; Wassef, L.; Narayanasamy, S.; Curley, R.W., Jr.; Harrison, E.H.; Hussain, M.M.; Quadro, L. Beta-apo-10′-carotenoids modulate placental microsomal triglyceride transfer protein expression and function to optimize transport of intact beta-carotene to the embryo. J. Biol. Chem. 2016, 291, 18525–18535. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J.; Hu, Y.; Noh, H.L.; Wei, J.; Huggins, L.A.; Rackmill, M.G.; Hamai, H.; Reid, B.N.; Blaner, W.S.; Huang, L.S. Decreased lipoprotein clearance is responsible for increased cholesterol in LDL receptor knockout mice with streptozotocin-induced diabetes. Diabetes 2008, 57, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Quadro, L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 2010, 652, 263–275. [Google Scholar] [PubMed]
- Quadro, L.; Blaner, W.S.; Salchow, D.J.; Vogel, S.; Piantedosi, R.; Gouras, P.; Freeman, S.; Cosma, M.P.; Colantuoni, V.; Gottesman, M.E. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999, 18, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wongsiriroj, N.; Blaner, W.S. The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surg. Nutr. 2014, 3, 126–139. [Google Scholar] [PubMed]
- Wassef, L.; Spiegler, E.; Quadro, L. Embryonic phenotype, beta-carotene and retinoid metabolism upon maternal supplementation of beta-carotene in a mouse model of severe vitamin A deficiency. Arch. Biochem. Biophys. 2013, 539, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D.; Cross, J.C. Development of structures and transport functions in the mouse placenta. Physiology 2005, 20, 180–193. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [PubMed]
- During, A.; Harrison, E.H. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal caco-2 cells. J. Lipid Res. 2007, 48, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Van Bennekum, A.; Werder, M.; Thuahnai, S.T.; Han, C.H.; Duong, P.; Williams, D.L.; Wettstein, P.; Schulthess, G.; Phillips, M.C.; Hauser, H. Class b scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 2005, 44, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Widjaja-Adhi, M.A.; Lobo, G.P.; Golczak, M.; Von Lintig, J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet. 2015, 24, 3206–3219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Harrison, E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Amengual, J.; Baus, D.; Shivdasani, R.A.; Taylor, D.; von Lintig, J. Genetics and diet regulate vitamin A production via the homeobox transcription factor isx. J. Biol. Chem. 2013, 288, 9017–9027. [Google Scholar] [CrossRef] [PubMed]
- Ribaya-Mercado, J.D.; Ordovas, J.M.; Russell, R.M. Effect of beta-carotene supplementation on the concentrations and distribution of carotenoids, vitamin E, vitamin A, and cholesterol in plasma lipoprotein and non-lipoprotein fractions in healthy older women. J. Am. Coll. Nutr. 1995, 14, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Shimano, H.; Hamilton, R.L.; Brown, M.S.; Goldstein, J.L. Disruption of LDL receptor gene in transgenic srebp-1a mice unmasks hyperlipidemia resulting from production of lipid-rich VLDL. J. Clin. Investig. 1999, 103, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Brewer, H.B., Jr. Abetalipoproteinemia. New insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease. JAMA 1993, 270, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Cohen, J.; Hobbs, H.H. Monogenic hypercholesterolemia: New insights in pathogenesis and treatment. J. Clin. Investig. 2003, 111, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Wassef, L.; Hamberger, L.; Piantedosi, R.; Palczewski, K.; Blaner, W.S.; Quadro, L. Retinyl ester formation by lecithin: Retinol acyltransferase (LRAT) is a key regulator of retinoid homeostasis in mouse embryogenesis. J. Biol. Chem. 2008, 283, 5611–5621. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E.; Tang, X.H.; Jessurun, J.; Gudas, L.J. Obesity leads to tissue, but not serum vitamin A deficiency. Sci. Rep. 2015, 5, 15893. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011, 25, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Umans, L.; Overbergh, L.; Serneels, L.; Tesseur, I.; Van Leuven, F. Analysis of expression of genes involved in apolipoprotein E-based lipoprotein metabolism in pregnant mice deficient in the receptor-associated protein, the low density lipoprotein receptor, or apolipoprotein E. Biol. Reprod. 1999, 61, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.S.; Kirk, E.A.; Rosenfeld, M.E.; LeBoeuf, R.C.; Chait, A. Dietary antioxidants inhibit development of fatty streak lesions in the LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Santos, R.D. Homozygous familial hypercholesterolemia: Current perspectives on diagnosis and treatment. Atherosclerosis 2012, 223, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, S.; Pisciotta, L.; Rabacchi, C.; Cefalu, A.B.; Noto, D.; Fasano, T.; Signori, A.; Fresa, R.; Averna, M.; Calandra, S. Spectrum of mutations and phenotypic expression in patients with autosomal dominant hypercholesterolemia identified in Italy. Atherosclerosis 2013, 227, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lye, S.H.; Chahil, J.K.; Bagali, P.; Alex, L.; Vadivelu, J.; Ahmad, W.A.; Chan, S.P.; Thong, M.K.; Zain, S.M.; Mohamed, R. Genetic polymorphisms in Ldlr, Apob, Pcsk9 and other lipid related genes associated with familial hypercholesterolemia in malaysia. PLoS ONE 2013, 8, e60729. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Olsen, R.H.; Merkens, L.S.; DeBarber, A.; Steiner, R.D.; Sullivan, P.M.; Maeda, N.; Raber, J. Apolipoprotein E-low density lipoprotein receptor interaction affects spatial memory retention and brain apoE levels in an isoform-dependent manner. Neurobiol. Dis. 2014, 64, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Huebbe, P.; Lange, J.; Lietz, G.; Rimbach, G. Dietary beta-carotene and lutein metabolism is modulated by the apoE genotype. Biofactors 2016, 42, 388–396. [Google Scholar] [CrossRef] [PubMed]

| Lipoprotein Fraction | TC (mg/dL) | TG (mg/dL) | BC (μg/dL) |
|---|---|---|---|
| Ldlr+/−+ BC | |||
| VLDL + Chylo | 10 ± 2 | 10 ± 3 | 9 ± 6 |
| LDL + IDL | 59 ± 13 | 10 ± 4 | 121 ± 95 |
| HDL | 92 ± 8 | 9 ± 1 | 384 ± 105 |
| Whole serum | 159 ± 14 | 27 ± 7 | 538 ± 190 |
| Ldlr−/−+ BC | |||
| VLDL + Chylo | 34 ± 11 * | 16 ± 3 * | 30 ± 16 * |
| LDL + IDL | 91 ± 16 * | 13 ± 2 | 265 ± 169 |
| HDL | 68 ± 50 | 12 ± 2 | 15; 402 |
| Whole serum | 208 ± 8 * | 30 ± 4 | 446 ± 107 |
| Maternal Genotype and Treatment | n | BC (μg/g) | ROH (μg/g) | RE (μg/g) |
|---|---|---|---|---|
| Ldlr+/− + Veh | 4 | n.d. | 7 ± 1 | 448 ± 171 |
| Ldlr+/− + BC | 7 | 104 ± 46 | 6 ± 1 | 856 ± 354 |
| Ldlr−/− + Veh | 4 | n.d. | 6 ± 3 | 686 ± 183 |
| Ldlr−/− + BC | 7 | 42 ± 23 * | 6 ± 1 | 649 ± 139 |
| Maternal Genotype and Treatment | Placental/Embryonic Genotype | n | Placental BC (ng/g) | Embryonic BC (ng/g) |
|---|---|---|---|---|
| Ldlr+/− + Veh | Ldlr+/− | 8 | n.d. | n.d. |
| Ldlr−/− | 6 | n.d. | n.d. | |
| Ldlr+/− + BC | Ldlr+/− | 13 | 581 ± 147 | 13 ± 5 |
| Ldlr−/− | 13 | 590 ± 158 | 18 ± 8 | |
| Ldlr−/− + Veh | Ldlr+/− | 10 | n.d. | n.d. |
| Ldlr−/− | 10 | n.d. | n.d. | |
| Ldlr−/− + BC | Ldlr+/− | 6 | 558 ± 187 | 19 ± 10 |
| Ldlr−/− | 6 | 529 ± 99 | 19 ± 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shete, V.; Costabile, B.K.; Kim, Y.-K.; Quadro, L. Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver. Nutrients 2016, 8, 765. https://doi.org/10.3390/nu8120765
Shete V, Costabile BK, Kim Y-K, Quadro L. Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver. Nutrients. 2016; 8(12):765. https://doi.org/10.3390/nu8120765
Chicago/Turabian StyleShete, Varsha, Brianna K. Costabile, Youn-Kyung Kim, and Loredana Quadro. 2016. "Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver" Nutrients 8, no. 12: 765. https://doi.org/10.3390/nu8120765




