Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. Materials
2.3. Cell Culture
2.4. Plasma 25(OH)D Measurement
2.5. Measurement of Cytokines
2.6. Flow Cytometry
2.7. Neutrophil Migration Assay
2.8. Statistics
3. Results
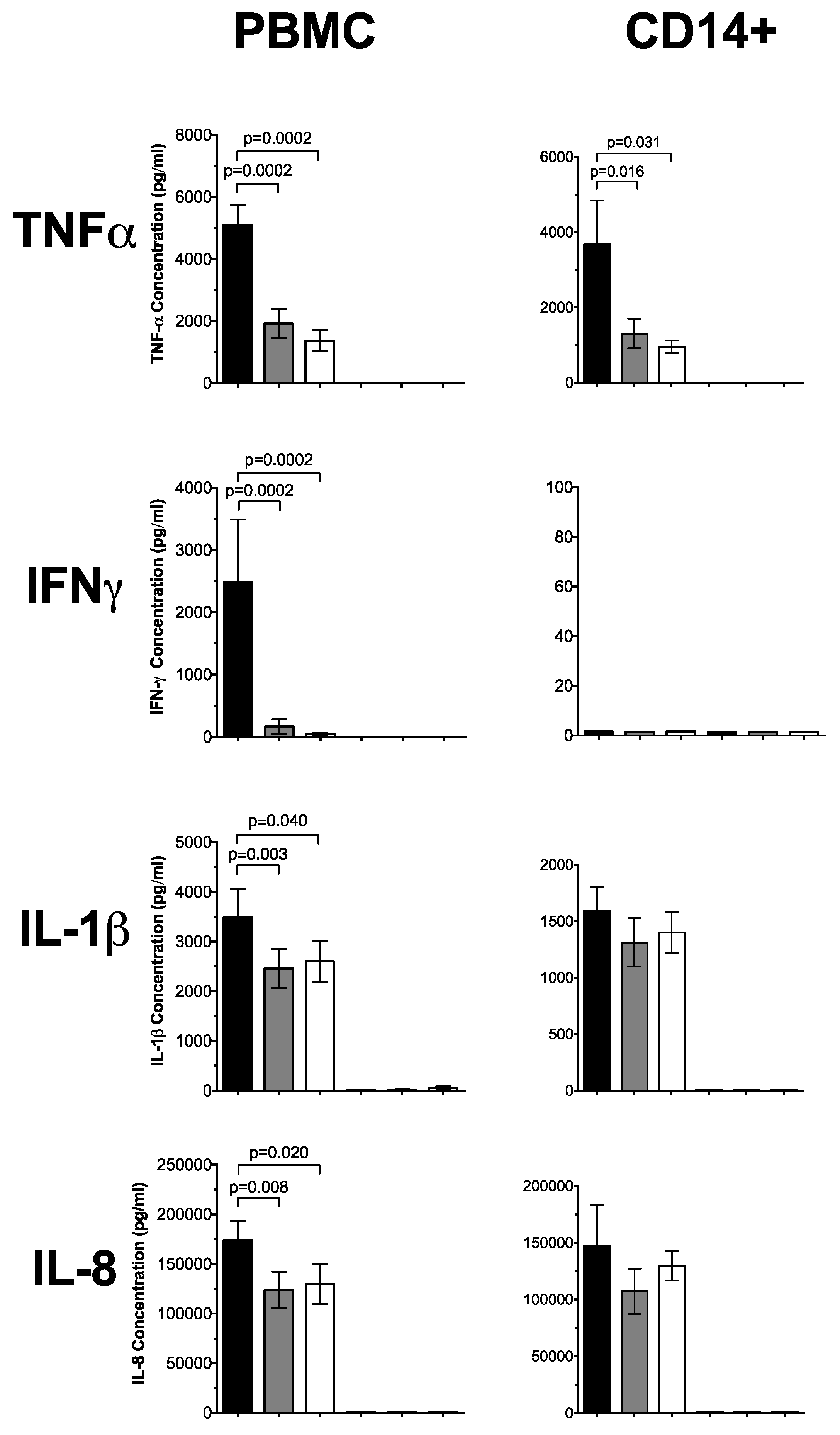
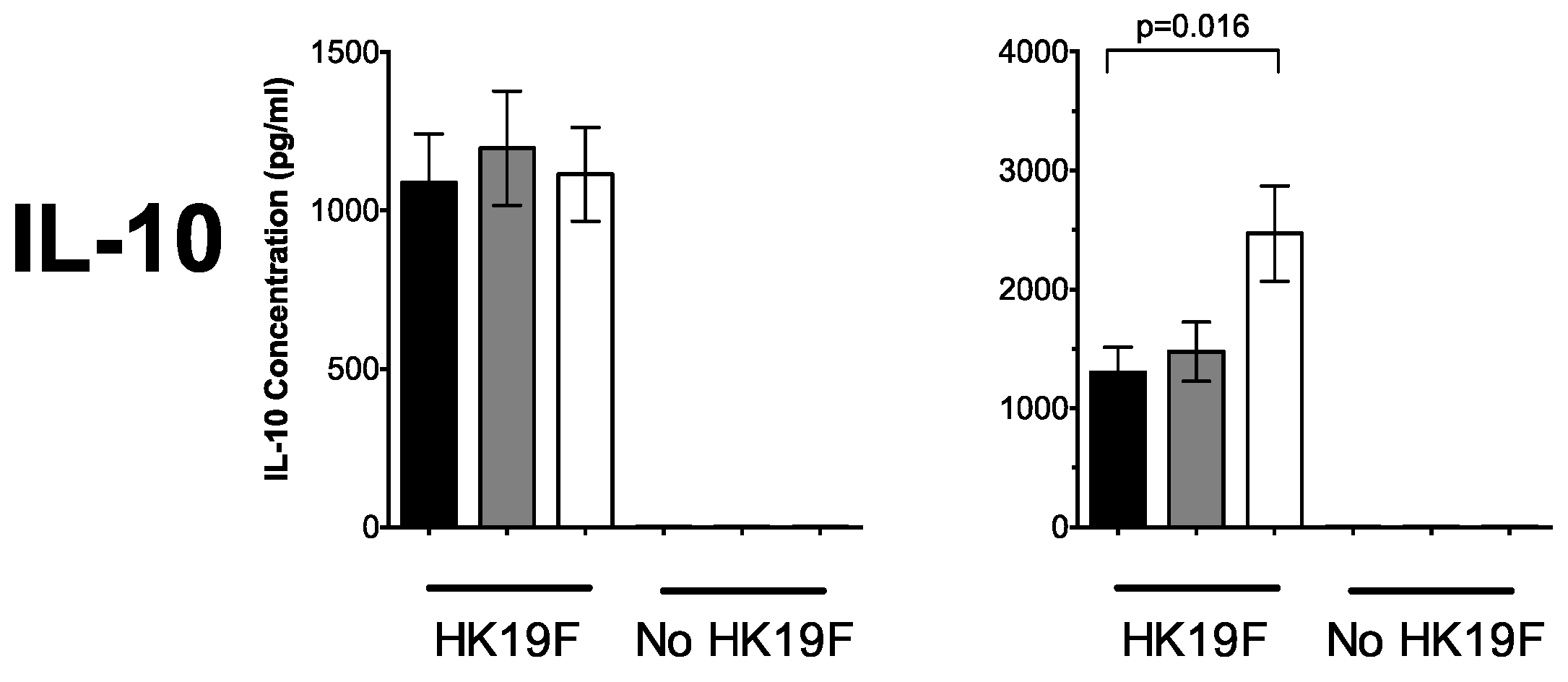
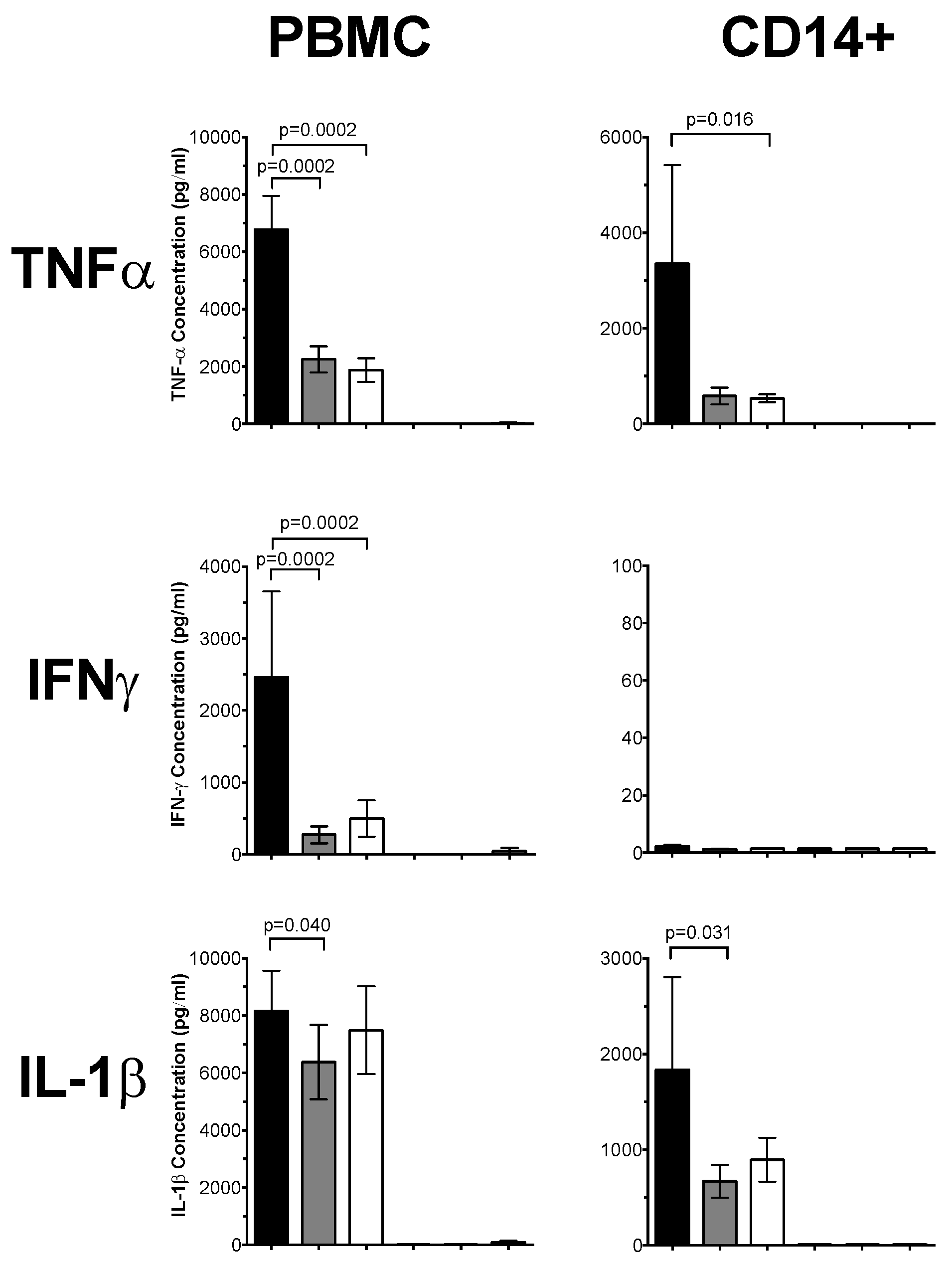
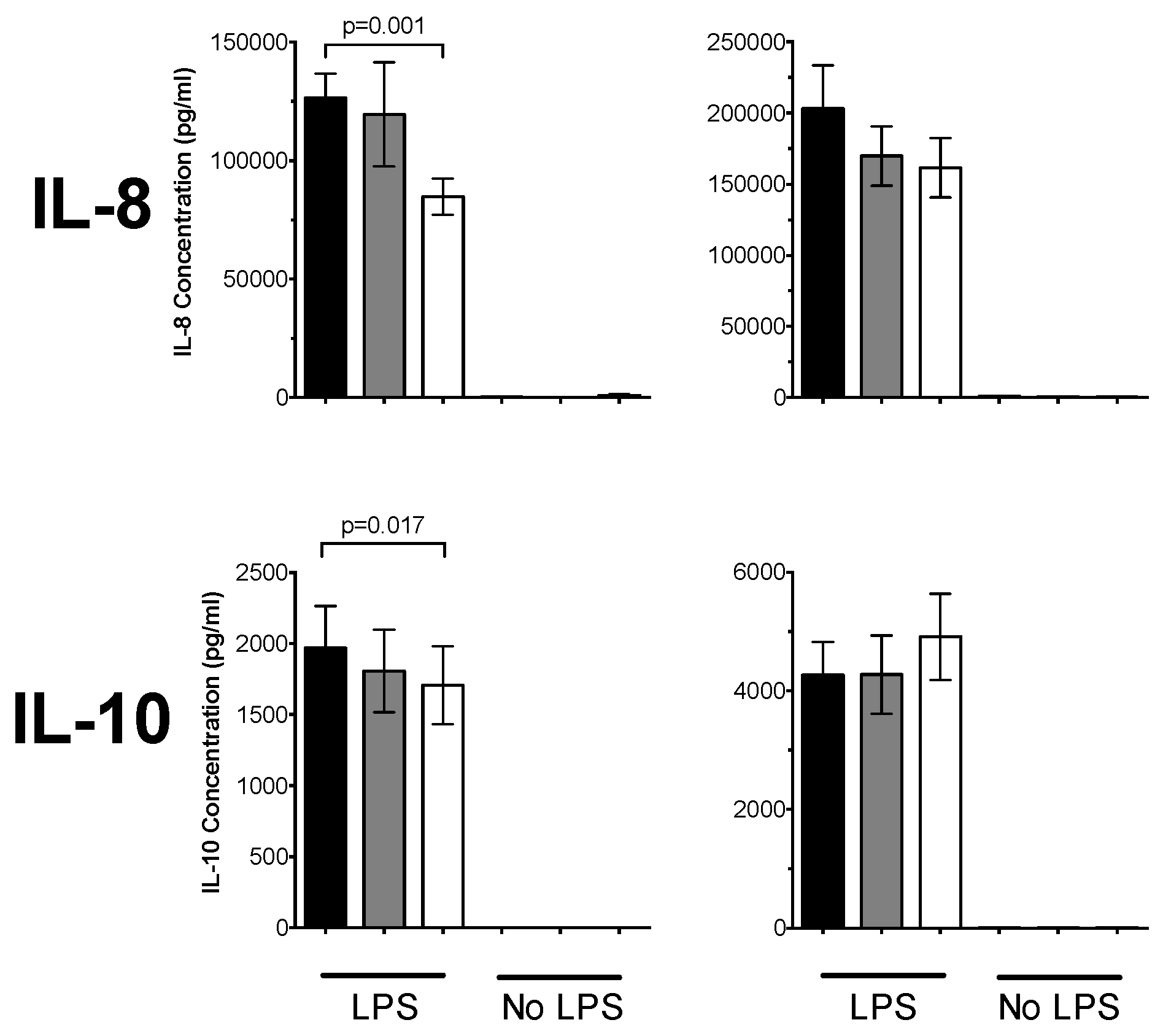
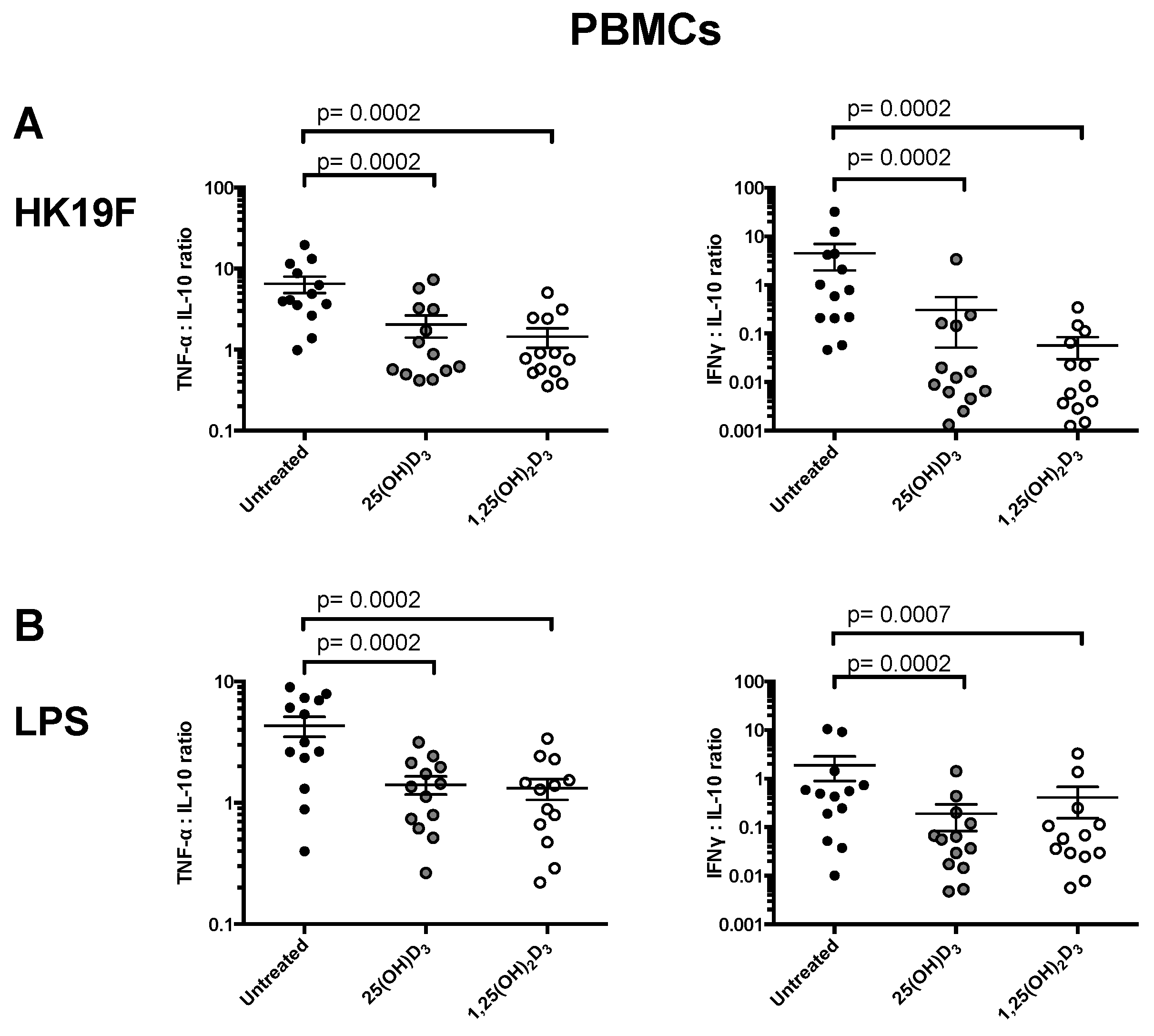
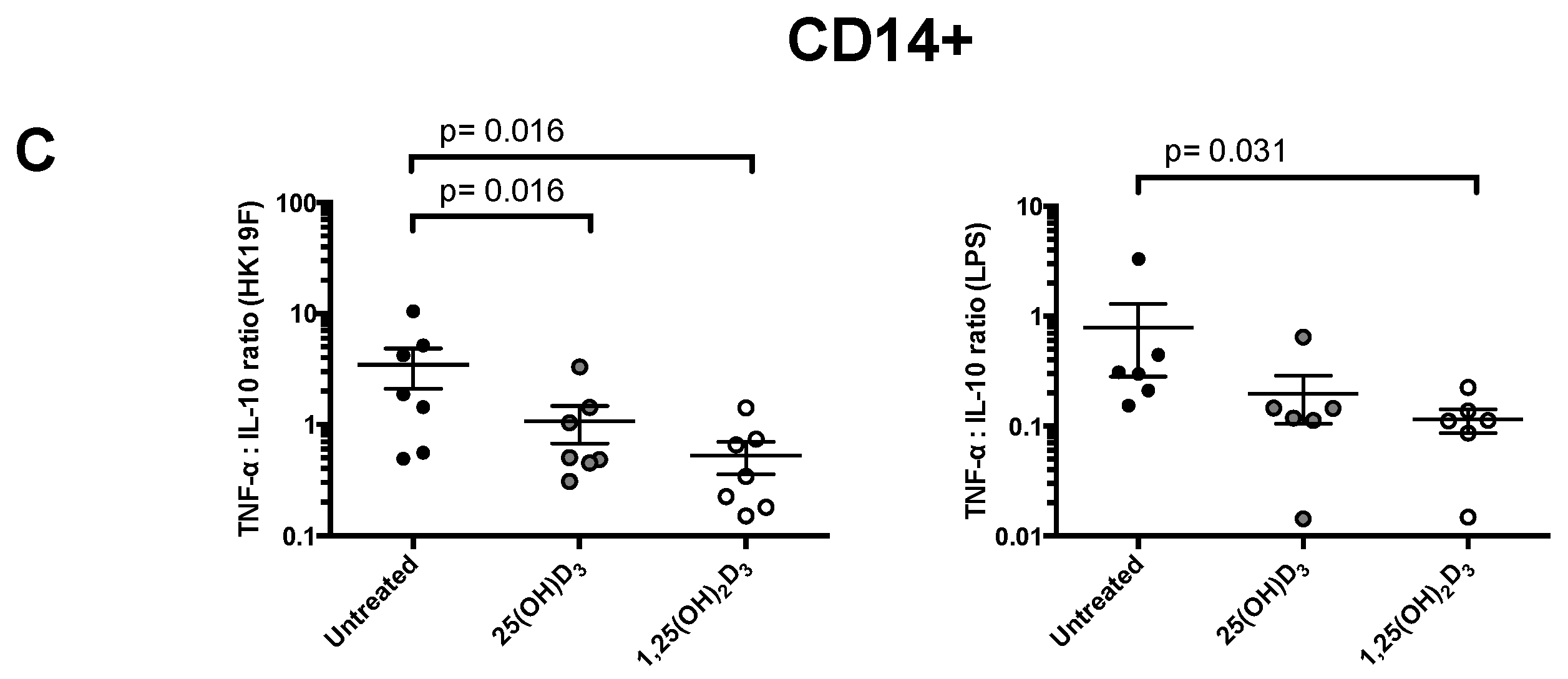
3.1. 1,25(OH)2D3 Suppresses Pro-Inflammatory Cytokines in PBMCs and CD14+ Monocytes
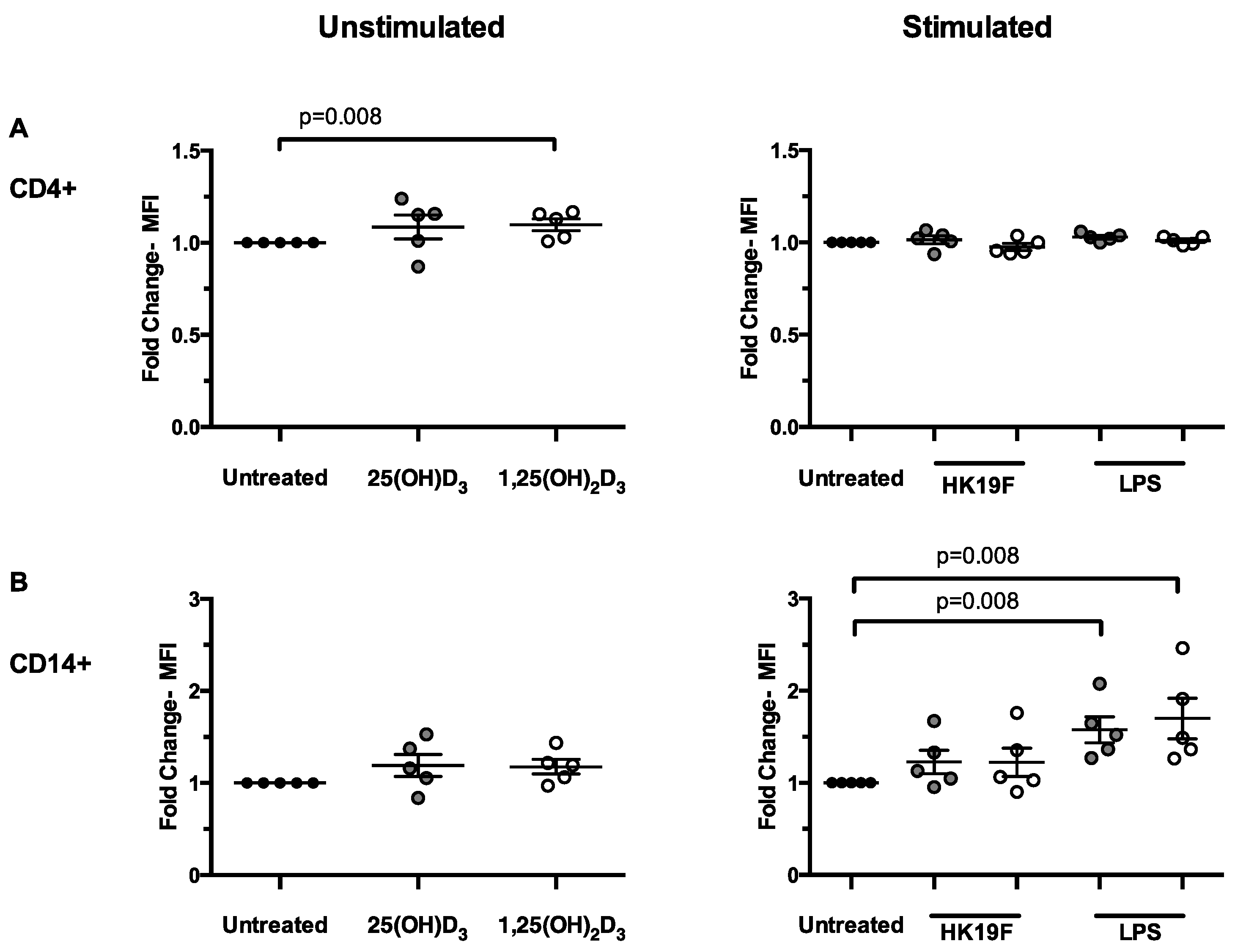
3.2. 1,25(OH)2D3 Enhances CD14 Expression in Stimulated PBMCs
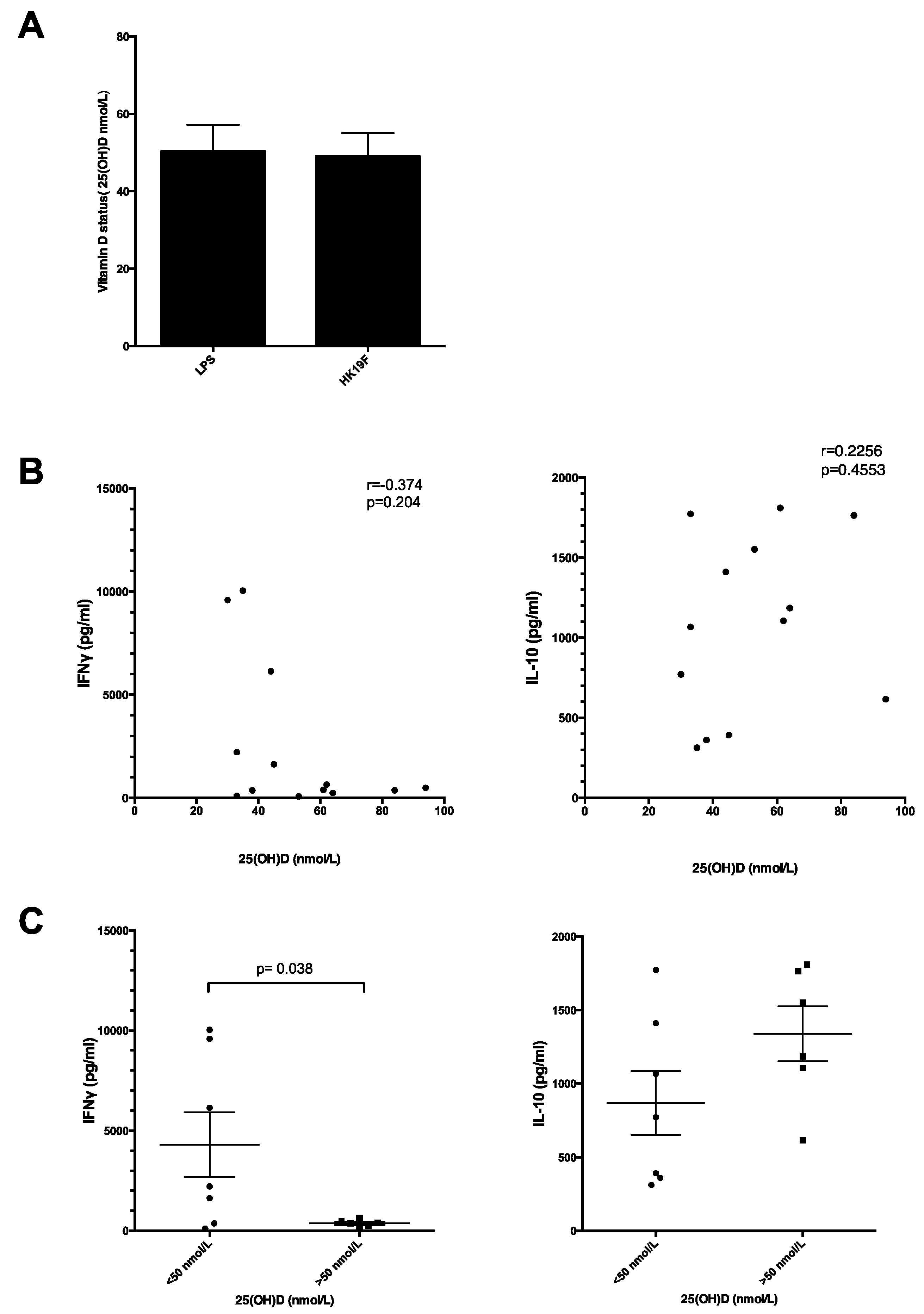
3.3. Plasma 25(OH)D Status Influences the Magnitude of Inflammatory Response
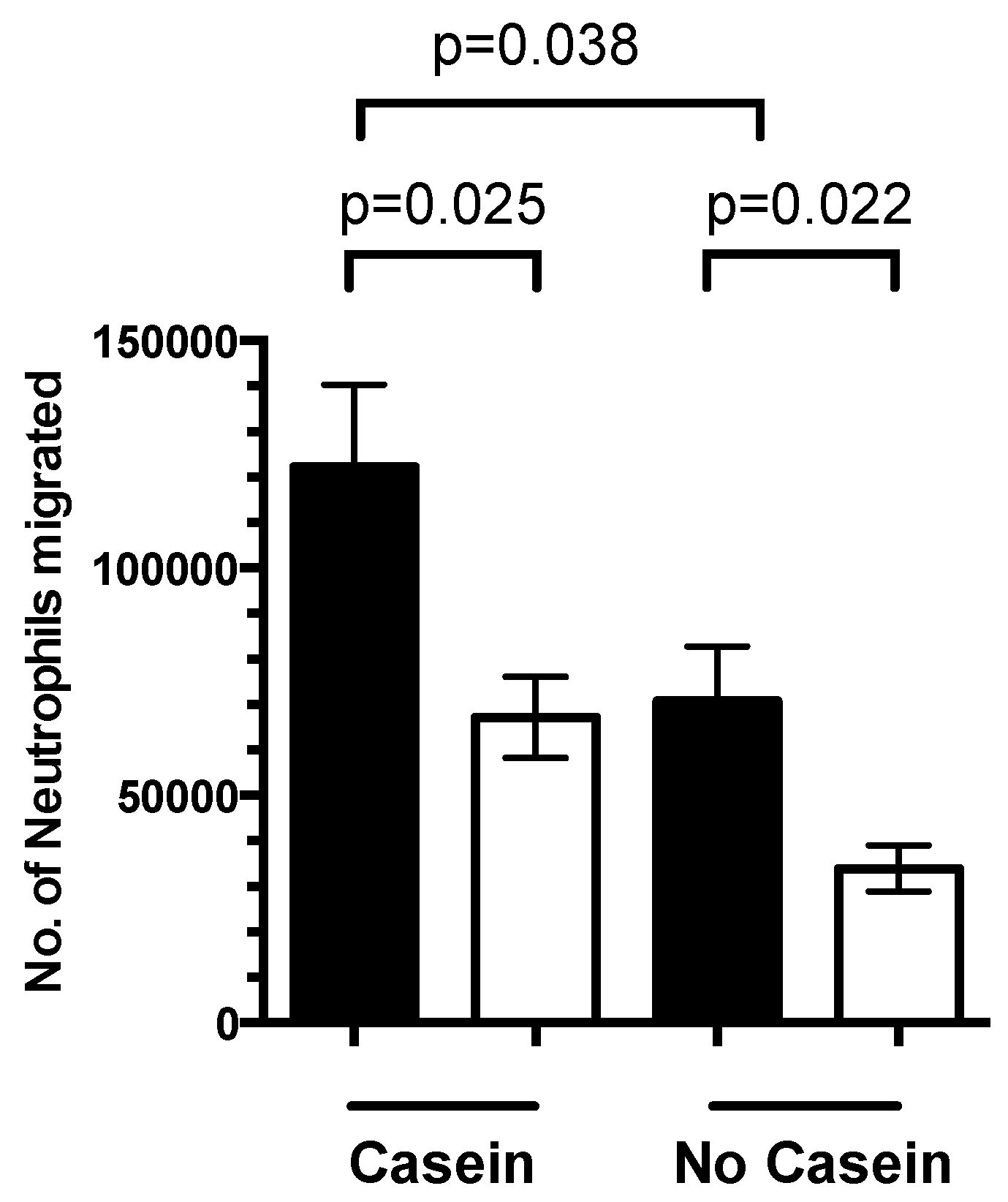
3.4. 1,25(OH)2D3 Regulates Neutrophil Migration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Petri, W.A., Jr.; Miller, M.; Binder, H.J.; Levine, M.M.; Dillingham, R.; Guerrant, R.L. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Investig. 2008, 118, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Lindh, A.U.; Bjorkhem-Bergman, L.; Lindh, J.D. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Hesketh, K.; Power, C.; Hypponen, E. Vitamin D status has a linear association with seasonal infections and lung function in british adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Shah, R.; Black, R.E.; Baqui, A.H. Vitamin D status and acute lower respiratory infection in early childhood in sylhet, bangladesh. Acta Paediatr. 2010, 99, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A., Jr.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef] [PubMed]
- Bryson, K.J.; Nash, A.A.; Norval, M. Does vitamin D protect against respiratory viral infections? Epidemiol. Infect. 2014, 142, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin D receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef] [PubMed]
- White, A.N.; Ng, V.; Spain, C.V.; Johnson, C.C.; Kinlin, L.M.; Fisman, D.N. Let the sun shine in: Effects of ultraviolet radiation on invasive pneumococcal disease risk in philadelphia, pennsylvania. BMC Infect. Dis. 2009, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Shevde, N.K. The vitamin D receptor. In Vitamin D, 2nd ed.; Feldman, F., Pike, J.W., Glorieux, F.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ojaimi, S.; Skinner, N.A.; Strauss, B.J.; Sundararajan, V.; Woolley, I.; Visvanathan, K. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: A pilot study. J. Transl. Med. 2013, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Izban, M.G.; Nowicki, B.J.; Nowicki, S. 1,25-dihydroxyvitamin D3 promotes a sustained lps-induced nf-kappab-dependent expression of cd55 in human monocytic thp-1 cells. PLoS ONE 2012, 7, e49318. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Olliver, M.; Spelmink, L.; Hiew, J.; Meyer-Hoffert, U.; Henriques-Normark, B.; Bergman, P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to streptococcus pneumoniae. J. Infect. Dis. 2013, 208, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I.; Galaktidou, G. Paricalcitol reduces basal and lipopolysaccharide-induced (lps) TNF-alpha and il-8 production by human peripheral blood mononuclear cells. Int. Urol. Nephrol. 2010, 42, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.L.; Chai, L.Y.; Koenen, H.J.; Sweep, F.C.; Joosten, I.; Netea, M.G.; van der Ven, A.J. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011, 164, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, peripheral blood mononuclear cells, and thp-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef] [PubMed]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting mapk phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Neme, A.; Nurminen, V.; Seuter, S.; Carlberg, C. The vitamin D-dependent transcriptome of human monocytes. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; de Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Malley, R.; Henneke, P.; Morse, S.C.; Cieslewicz, M.J.; Lipsitch, M.; Thompson, C.M.; Kurt-Jones, E.; Paton, J.C.; Wessels, M.R.; Golenbock, D.T. Recognition of pneumolysin by toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 2003, 100, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kang, L.; Yao, H.; He, Y.; Wang, X.; Xu, W.; Song, Z.; Yin, Y.; Zhang, X. Streptococcus pneumoniae endopeptidase O (PepO) elicits a strong innate immune response in mice via TLR2 and TLR4 signaling pathways. Front. Cell. Infect. Microbiol. 2016, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, G.; Chimalapati, S.; Pollard, T.; Lapp, T.; Cohen, J.; Camberlein, E.; Stafford, S.; Periselneris, J.; Aldridge, C.; Vollmer, W.; et al. Tlr-mediated inflammatory responses to streptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins. J. Immunol. 2014, 193, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Reading, P.C.; Wang, N.; Diavatopoulos, D.A.; Wijburg, O.L. Increased nasopharyngeal bacterial titers and local inflammation facilitate transmission of streptococcus pneumoniae. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Valls Seron, M.; Duitman, J.; Geldhoff, M.; Engelen-Lee, J.; Havik, S.R.; Brouwer, M.C.; van de Beek, D.; Spek, C.A. CCAAT/enhancer-binding protein δ (C/EBPδ) aggravates inflammation and bacterial dissemination during pneumococcal meningitis. J. Neuroinflamm. 2015, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Antunes, G.; Evans, S.A.; Lordan, J.L.; Frew, A.J. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 2002, 20, 990–995. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.; Yu, S.; Ooi, J.H.; Cantorna, M.T. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int. Immunol. 2011, 23, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Waddell, A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014, 1317, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.E.; Coleman, L.A. Vitamin D and influenza. Adv. Nutr. 2012, 3, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.; Muris, A.H.; Hupperts, R.; Damoiseaux, J. Vitamin D effects on B cell function in autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Carvalho, C.; Boleixa, D.; Bettencourt, A.; Leal, B.; Guimaraes, J.; Neves, E.; Oliveira, J.C.; Almeida, I.; Farinha, F.; et al. Vitamin D supplementation effects on FoxP3 expression in T cells and FoxP3+/IL-17A ratio and clinical course in systemic lupus erythematosus patients: A study in a portuguese cohort. Immunol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabrysova, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1alpha,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct foxp3+ and il-10+ cd4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Christopher, K.B. Vitamin D and critical illness outcomes. Curr. Opin. Crit. Care 2016, 22, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Grainger, J.R.; Askenase, M.H.; Guimont-Desrochers, F.; da Fonseca, D.M.; Belkaid, Y. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol. Rev. 2014, 259, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Dembinski, J.; Stehle, P. Low vitamin D status is associated with low cord blood levels of the immunosuppressive cytokine interleukin-10. Pediatr. Allergy Immunol. 2004, 15, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kruit, A.; Zanen, P. The association between vitamin D and C-reactive protein levels in patients with inflammatory and non-inflammatory diseases. Clin. Biochem. 2016, 49, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.; Patel, J.M.; Scott, A.; Lax, S.; Dancer, R.C.; D’Souza, V.; Greenwood, H.; Fraser, W.D.; Gao, F.; Sapey, E.; et al. Vitamin D deficiency in human and murine sepsis. Crit. Care Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.E.; Bosco, A.; Troy, N.M.; Gorman, S.; Hart, P.H.; Kicic, A.; Zosky, G.R. Identification of genes differentially regulated by vitamin D deficiency that alter lung pathophysiology and inflammation in allergic airways disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L653–L663. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zugel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Verway, M.; Bouttier, M.; Wang, T.T.; Carrier, M.; Calderon, M.; An, B.S.; Devemy, E.; McIntosh, F.; Divangahi, M.; Behr, M.A.; et al. Vitamin D induces interleukin-1beta expression: Paracrine macrophage epithelial signaling controls m. Tuberculosis infection. PLoS Pathog. 2013, 9, e1003407. [Google Scholar] [CrossRef] [PubMed]
- Science, M.; Maguire, J.L.; Russell, M.L.; Smieja, M.; Walter, S.D.; Loeb, M. Serum 25-hydroxyvitamin D level and influenza vaccine immunogenicity in children and adolescents. PLoS ONE 2014, 9, e83553. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Drozdenko, G.; Lahl, A.; Unterwalder, N.; Mei, H.; Volk, H.D.; Dorner, T.; Radbruch, A.; Worm, M. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur. J. Clin. Nutr. 2011, 65, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.P.; Dragunsky, E.M.; Chumakov, K.M. 1,25-dihydroxyvitamin D3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J. Infect. Dis. 2006, 193, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Archer, F.E.; Joshi-Kale, M.; Vetrano, A.M.; Weinberger, B. Decreased anti-inflammatory responses to vitamin D in neonatal neutrophils. Mediat. Inflamm. 2011, 2011, 598345. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, W.M.; Akbar, N.A.; Zacharek, M.A. Vitamin D and chronic rhinitis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.M.; Gwyer Findlay, E.; McFarlane, A.J.; Fitch, P.M.; Bottcher, B.; Colegrave, N.; Paras, A.; Jozwik, A.; Chiu, C.; Schwarze, J.; et al. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J. Immunol. 2016, 196, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.; Alyahya, K.O.; Raghupathy, R. Association between levels of vitamin D and inflammatory markers in healthy women. J. Inflamm. Res. 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Dopico, X.C.; Evangelou, M.; Ferreira, R.C.; Guo, H.; Pekalski, M.L.; Smyth, D.J.; Cooper, N.; Burren, O.S.; Fulford, A.J.; Hennig, B.J.; et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 2015, 6, 7000. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.E.; Collinson, A.C.; Fulford, A.J.; Jalil, F.; Siegrist, C.A.; Goldblatt, D.; Hanson, L.A.; Prentice, A.M. Effect of month of vaccine administration on antibody responses in the Gambia and Pakistan. Trop. Med. Int. Health 2006, 11, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Lalor, M.K.; Ben-Smith, A.; Gorak-Stolinska, P.; Weir, R.E.; Floyd, S.; Blitz, R.; Mvula, H.; Newport, M.J.; Branson, K.; McGrath, N.; et al. Population differences in immune responses to bacille calmette-guerin vaccination in infancy. J. Infect. Dis. 2009, 199, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.X.; Zhou, Q.F.; Xu, Z.W.; Hao, J.H.; Huang, K.; Mou, Z.; Jiang, X.M.; Tao, F.B.; Zhu, P. Inverse correlation between vitamin D and C-reactive protein in newborns. Nutrients 2015, 7, 9218–9228. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Vong, L.; Pinnell, L.J.; Rautava, J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency predisposes to adherent-invasive escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflamm. Bowel Dis. 2015, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Meckel, K.; Li, Y.C.; Lim, J.; Kocherginsky, M.; Weber, C.; Almoghrabi, A.; Chen, X.; Kaboff, A.; Sadiq, F.; Hanauer, S.B.; et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am. J. Clin. Nutr. 2016, 104, 113–120. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoe, E.; Nathanielsz, J.; Toh, Z.Q.; Spry, L.; Marimla, R.; Balloch, A.; Mulholland, K.; Licciardi, P.V. Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection. Nutrients 2016, 8, 806. https://doi.org/10.3390/nu8120806
Hoe E, Nathanielsz J, Toh ZQ, Spry L, Marimla R, Balloch A, Mulholland K, Licciardi PV. Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection. Nutrients. 2016; 8(12):806. https://doi.org/10.3390/nu8120806
Chicago/Turabian StyleHoe, Edwin, Jordan Nathanielsz, Zheng Quan Toh, Leena Spry, Rachel Marimla, Anne Balloch, Kim Mulholland, and Paul V. Licciardi. 2016. "Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection" Nutrients 8, no. 12: 806. https://doi.org/10.3390/nu8120806




