Phenotypic Stability of Energy Balance Responses to Experimental Total Sleep Deprivation and Sleep Restriction in Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.4. Statistical Analyses
3. Results
3.1. Participant Characteristics
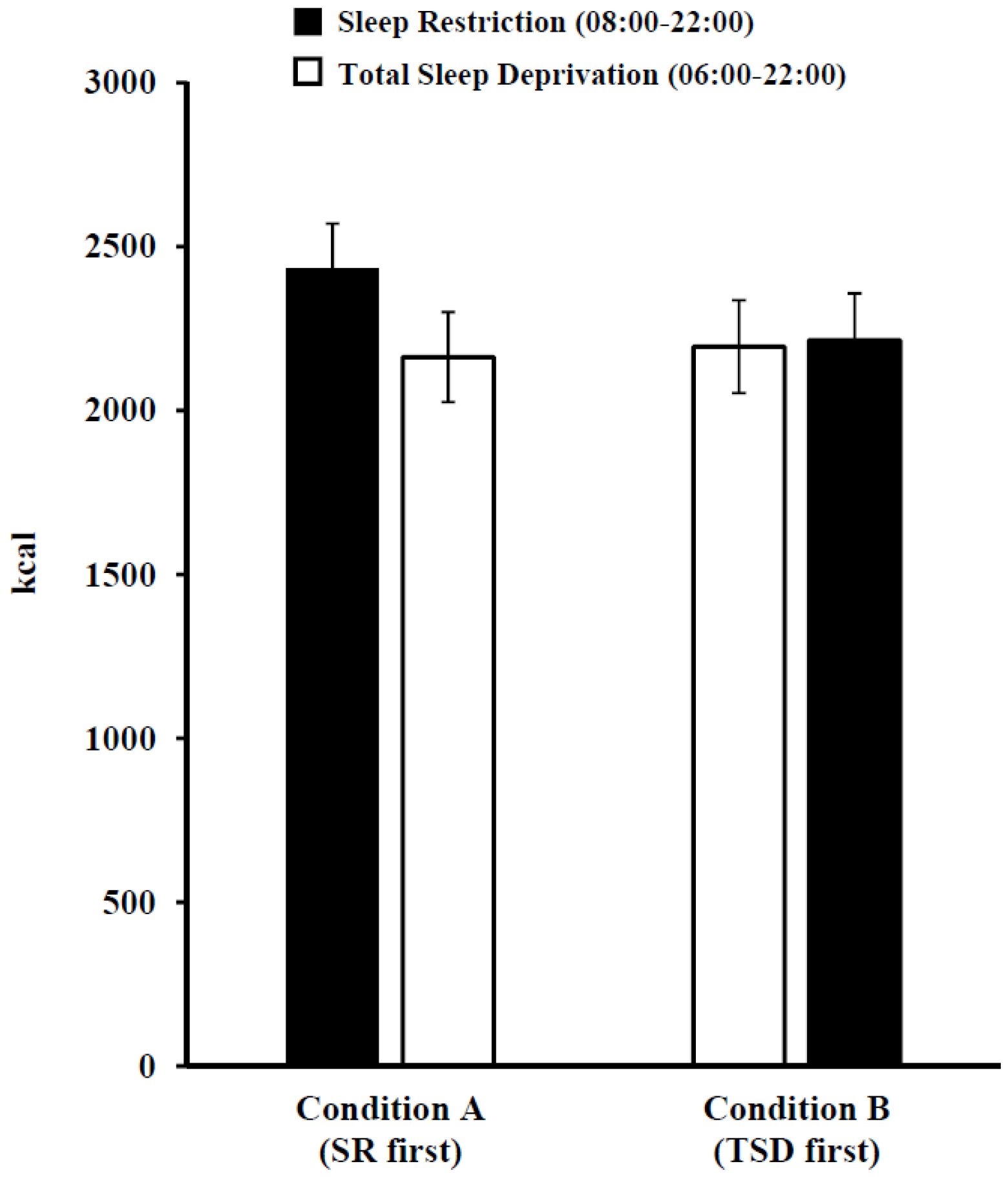
3.2. Late-Night Intake during Sleep Restriction and Total Sleep Deprivation
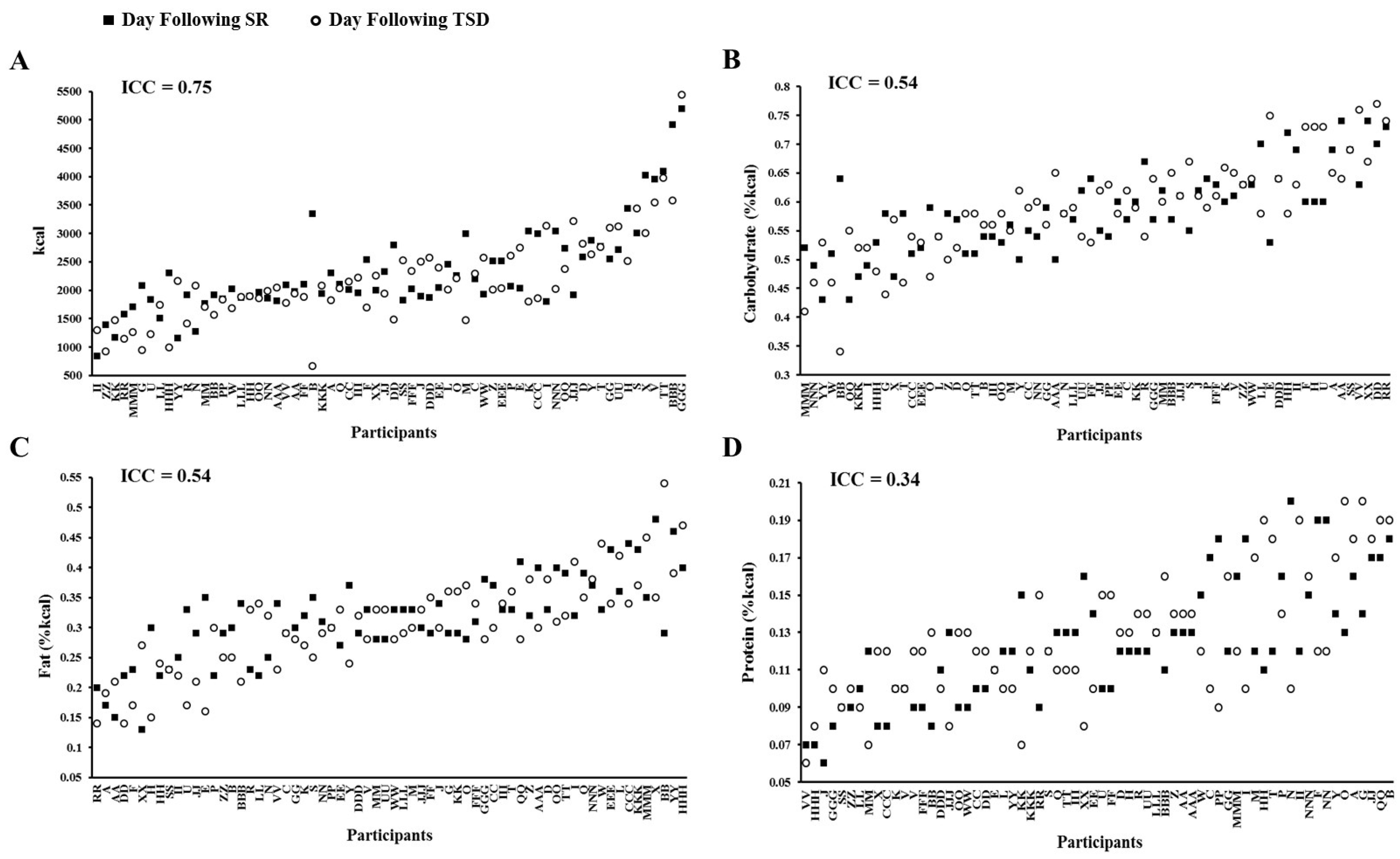
3.3. Daily Intake Following Sleep Restriction and Total Sleep Deprivation
3.4. Recovery Sleep Following Sleep Restriction or Total Sleep Deprivation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013, 36, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Hinrichs, S.; Jauch-Chara, K.; Hitze, B.; Later, W.; Wilms, B.; Settler, U.; Peters, A.; Kiosz, D.; Muller, M.J. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes. Facts 2008, 1, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Brondel, L.; Romer, M.A.; Nougues, P.M.; Touyarou, P.; Davenne, D. Acute partial sleep deprivation increases food intake in healthy men. Am. J. Clin. Nutr. 2010, 91, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Roberts, A.L.; Chen, J.; Kelleman, M.; O’Keeffe, M.; RoyChoudhury, A.; Jones, P.J. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am. J. Clin. Nutr. 2011, 94, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Calvin, A.D.; Carter, R.E.; Adachi, T.; Macedo, P.G.; Albuquerque, F.N.; van der Walt, C.; Bukartyk, J.; Davison, D.E.; Levine, J.A.; Somers, V.K. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013, 144, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.L.; Kilkus, J.M.; Delebecque, F.; Abraham, V.; Day, A.; Whitmore, H.R.; Tasali, E. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity 2016, 24, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Capers, P.L.; Fobian, A.D.; Kaiser, K.A.; Borah, R.; Allison, D.B. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev. 2015, 16, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am. J. Clin. Nutr. 2014, 100, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Kasza, K.; Schoeller, D.A.; Penev, P.D. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 2009, 89, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Spaeth, A.M.; Ma, N.; Zhu, S.; Hu, S.; Goel, N.; Detre, J.A.; Dinges, D.F.; Rao, H. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci. Rep. 2015, 5, 8215. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Nilsson, E.K.; Nilsson, V.C.; Cedernaes, J.; Rångtell, F.H.; Vogel, H.; Dickson, S.L.; Broman, J.E.; Hogenkamp, P.S.; Schiöth, H.B.; et al. Acute sleep deprivation increases food purchasing in men. Obesity 2013, 21, E555–E560. [Google Scholar] [CrossRef] [PubMed]
- Hogenkamp, P.S.; Nilsson, E.; Nilsson, V.C.; Chapman, C.D.; Vogel, H.; Lundberg, L.S.; Zarei, S.; Cedernaes, J.; Rångtell, F.H.; Broman, J.E.; et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology 2013, 38, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Rising, R.; Albu, J.B.; St-Onge, M.P. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am. J. Clin. Nutr. 2013, 98, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.M.; Melanson, E.L.; Frydendall, E.J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 2011, 589, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Al Khatib, H.K.; Harding, S.V.; Darzi, J.; Pot, G.K. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.L.; Wesensten, N.J.; Balkin, T.J. Trait-like vulnerability to total and partial sleep loss. Sleep 2012, 35, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, H.P.A.; Baynard, M.D.; Maislin, G.; Dinges, D.F. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep 2004, 27, 423–433. [Google Scholar] [PubMed]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Phenotypic vulnerability of energy balance responses to sleep loss in healthy adults. Sci. Rep. 2015, 5, 14920. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Resting metabolic rate varies by race and by sleep duration. Obesity 2015, 23, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; Van Dongen, H.P.A.; Maislin, G.; Dinges, D.F. Neurobehavioral dynamics following chronic sleep restriction: Dose-response effects of one night for recovery. Sleep 2010, 33, 1013–1026. [Google Scholar] [PubMed]
- Axelsson, J.; Kecklund, G.; Åkerstedt, T.; Donofrio, P.; Lekander, M.; Ingre, M. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol. Int. 2008, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Haavisto, M.L.; Porkka-Heiskanen, T.; Hublin, C.; Härmä, M.; Mutanen, P.; Müller, K.; Virkkala, J.; Sallinen, M. Sleep restriction for the duration of a work week impairs multitasking performance. J. Sleep Res. 2010, 19, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, M.; Onninen, J.; Tirkkonen, K.; Haavisto, M.L.; Härmä, M.; Kubo, T.; Mutanen, P.; Virkkala, J.; Tolvanen, A.; Porkka-Heiskanen, T. Effects of cumulative sleep restriction on self-perceptions while multitasking. J. Sleep Res. 2013, 22, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.; Sagaspe, P.; Prague, M.; Tassi, P.; Capelli, A.; Bioulac, B.; Commenges, D.; Taillard, J. Acute versus chronic partial sleep deprivation in middle-aged people: Differential effect on performance and sleepiness. Sleep 2012, 35, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Pejovic, S.; Basta, M.; Vgontzas, A.N.; Kritikou, I.; Shaffer, M.L.; Tsaoussoglou, M.; Stiffler, D.; Stefanakis, Z.; Bixler, E.O.; Chrousos, G.P. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E890–E896. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Dement, W.C. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology 1981, 18, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lamond, N.; Jay, S.M.; Dorrian, J.; Ferguson, S.A.; Jones, C.; Dawson, D. The dynamics of neurobehavioural recovery following sleep loss. J. Sleep Res. 2007, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Belenky, G.; Wesensten, N.J.; Thorne, D.R.; Thomas, M.L.; Sing, H.C.; Redmond, D.P.; Russo, M.B.; Balkin, T.J. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J. Sleep Res. 2003, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P. Impact of sleep duration on food intake regulation: Different mechanisms by sex? Obesity 2016, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.S.; Reilly, C.; Midkiff, K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 1989, 74, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Hise, M.E.; Sullivan, D.K.; Jacobsen, D.J.; Johnson, S.L.; Donnelly, J.E. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am. J. Clin. Nutr. 2002, 75, 263–267. [Google Scholar] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.L.; Wesensten, N.J.; Bliese, P.D.; Balkin, T.J. Banking sleep: Realization of benefits during subsequent sleep restriction and recovery. Sleep 2009, 32, 311–321. [Google Scholar] [PubMed]
- McNeil, J.; Doucet, É.; Brunet, J.F.; Hintze, L.J.; Chaumont, I.; Langlois, É.; Maitland, R.; Riopel, A.; Forest, G. The effects of sleep restriction and altered sleep timing on energy intake and energy expenditure. Physiol. Behav. 2016, 164, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J.; Kern, A.S.; Zee, P.C. Role of sleep timing in caloric intake and BMI. Obesity 2011, 19, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Bormes, A.; Salazar, I. The role of sleep duration on energy balance: An update. Curr. Nutr. Rep. 2016, 5, 278–285. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of diet on sleep quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Objective measurements of energy balance are associated with sleep architecture in healthy adults. Sleep 2016, in press. [Google Scholar]
- Smith, S.L.; Ludy, M.J.; Tucker, R.M. Changes in taste preference and steps taken after sleep curtailment. Physiol. Behav. 2016, 163, 228–233. [Google Scholar] [CrossRef] [PubMed]


| N | Age (Years) | BMI (kg/m2) | Women | African American | Chronotype a | Sleep Duration (h) b | Sleep Midpoint (Time ± h) b | |
|---|---|---|---|---|---|---|---|---|
| All Participants | 66 | 34.4 ± 9.0 | 24.4 ± 3.2 | 32 (48.5%) | 48 (72.7%) | 42.1 ± 5.9 | 8.0 ± 0.5 | 03:34 ± 0.8 |
| Condition A (SR first) | 34 | 33.2 ± 8.9 | 24.6 ± 3.0 | 16 (47.1%) | 24 (70.6%) | 40.9 ± 6.1 | 8.1 ± 0.4 | 03:41 ± 0.8 |
| Condition B (TSD first) | 32 | 35.6 ± 9.1 | 24.2 ± 3.3 | 16 (50.0%) | 24 (75.0%) | 43.6 ± 5.3 | 8.0 ± 0.6 | 03:27 ± 0.8 |
| Day Following SR (08:00–22:00; N = 66) | Day Following TSD (06:00–22:00; N = 66) | p Values | |
|---|---|---|---|
| Kcal | 2326.6 ± 811.1 | 2178.9 ± 793.7 | 0.11 |
| Protein (%kcal) | 12.4 ± 3.3 | 12.8 ± 3.4 | 0.50 |
| Carbohydrate (%kcal) | 58.4 ± 7.3 | 59.0 ± 8.6 | 0.56 |
| Fat (%kcal) | 31.2 ± 7.1 | 30.4 ± 8.1 | 0.31 |
| Sugar (%kcal) | 28.7 ± 7.5 | 28.4 ± 8.8 | 0.94 |
| Saturated Fat (%kcal) | 11.0 ± 3.5 | 10.0 ± 3.5 | 0.03 |
| Fiber (g) | 19.6 ± 9.6 | 18.5 ± 10.0 | 0.38 |
| R1 | R2 | R3 | R4 | |||||
|---|---|---|---|---|---|---|---|---|
| SR | TSD | SR | TSD | SR | TSD | SR | TSD | |
| Kcal | 2307.9 ± 615.0 † | 2007.4 ± 659.0 | 2055.3 ± 607.7 | 2060.1 ± 654.0 | 2096.2 ± 761.6 | 2018.7 ± 751.9 | 2026.6 ± 863.8 | 1861.2 ± 630.0 |
| Protein (%kcal) | 10.8 ± 3.3 | 16.2 ± 5.0 * | 12.7 ± 4.8 | 17.6 ± 3.3 | 11.8 ± 3.8 | 16.2 ± 5.0 | 15.0 ± 7.8 | 15.1 ± 3.8 |
| Carbohydrate (%kcal) | 63.1 ± 6.0 | 55.2 ± 10.0 | 61.4 ± 10.2 | 52.7 ± 11.1 | 62.6 ± 8.6 | 57.8 ± 8.0 | 56.7 ± 13.0 | 53.0 ± 9.5 |
| Sugar (%kcal) | 31.2 ± 6.9 | 24.1 ± 9.6 | 29.0 ± 9.9 | 23.2 ± 4.6 | 34.9 ± 12.6 | 27.7 ± 7.2 | 26.8 ± 7.6 | 26.9 ± 6.6 |
| Fat (%kcal) | 28.1 ± 5.6 | 30.0 ± 7.6 | 27.8 ± 7.3 | 31.8 ± 8.8 | 27.4 ± 7.6 | 28.3 ± 7.7 | 31.7 ± 6.8 | 33.5 ± 7.6 |
| Saturated Fat (%kcal) | 10.4 ± 3.1 | 11.9 ± 3.4 | 10.1 ± 4.1 | 12.7 ± 4.5 | 8.5 ± 2.9 | 11.5 ± 4.4 | 10.7 ± 3.4 | 12.6 ± 4.2 |
| Fiber (g) | 20.7 ± 7.1 | 21.4 ± 9.3 | 17.7 ± 11.1 | 19.0 ± 10.4 | 12.0 ± 5.7 | 21.1 ± 15.5 | 15.1 ± 6.3 | 16.8 ± 9.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, L.E.; Spaeth, A.M.; Goel, N. Phenotypic Stability of Energy Balance Responses to Experimental Total Sleep Deprivation and Sleep Restriction in Healthy Adults. Nutrients 2016, 8, 823. https://doi.org/10.3390/nu8120823
Dennis LE, Spaeth AM, Goel N. Phenotypic Stability of Energy Balance Responses to Experimental Total Sleep Deprivation and Sleep Restriction in Healthy Adults. Nutrients. 2016; 8(12):823. https://doi.org/10.3390/nu8120823
Chicago/Turabian StyleDennis, Laura E., Andrea M. Spaeth, and Namni Goel. 2016. "Phenotypic Stability of Energy Balance Responses to Experimental Total Sleep Deprivation and Sleep Restriction in Healthy Adults" Nutrients 8, no. 12: 823. https://doi.org/10.3390/nu8120823





