Low Prevalence of Vitamin D Insufficiency among Nepalese Infants Despite High Prevalence of Vitamin D Insufficiency among Their Mothers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Clearance
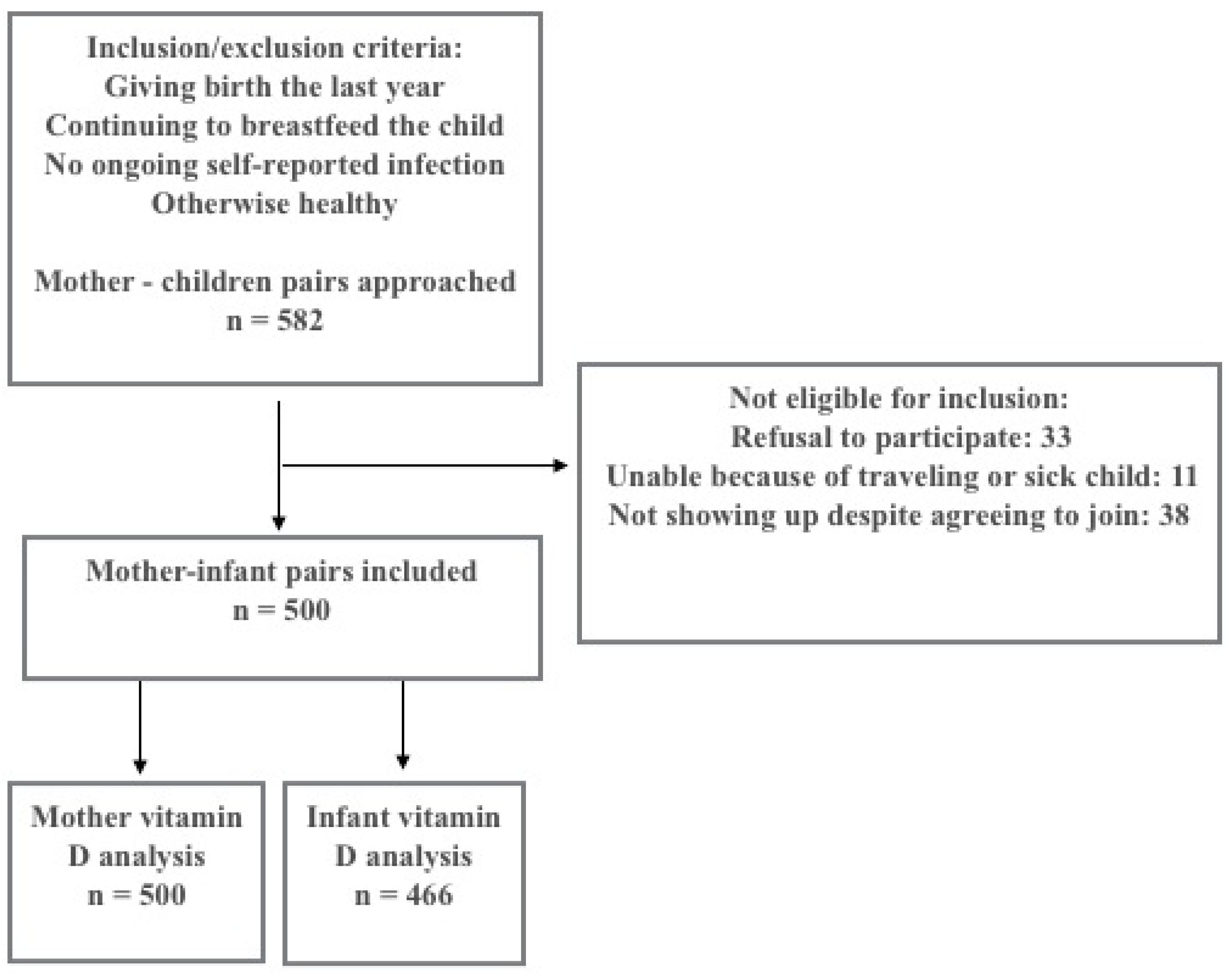
2.2. Study Design and Participants
2.3. Data Collection
2.4. 25(OH)D Analysis and Other Laboratory Tests
2.5. Outcome Variables and Definition of Vitamin D Status
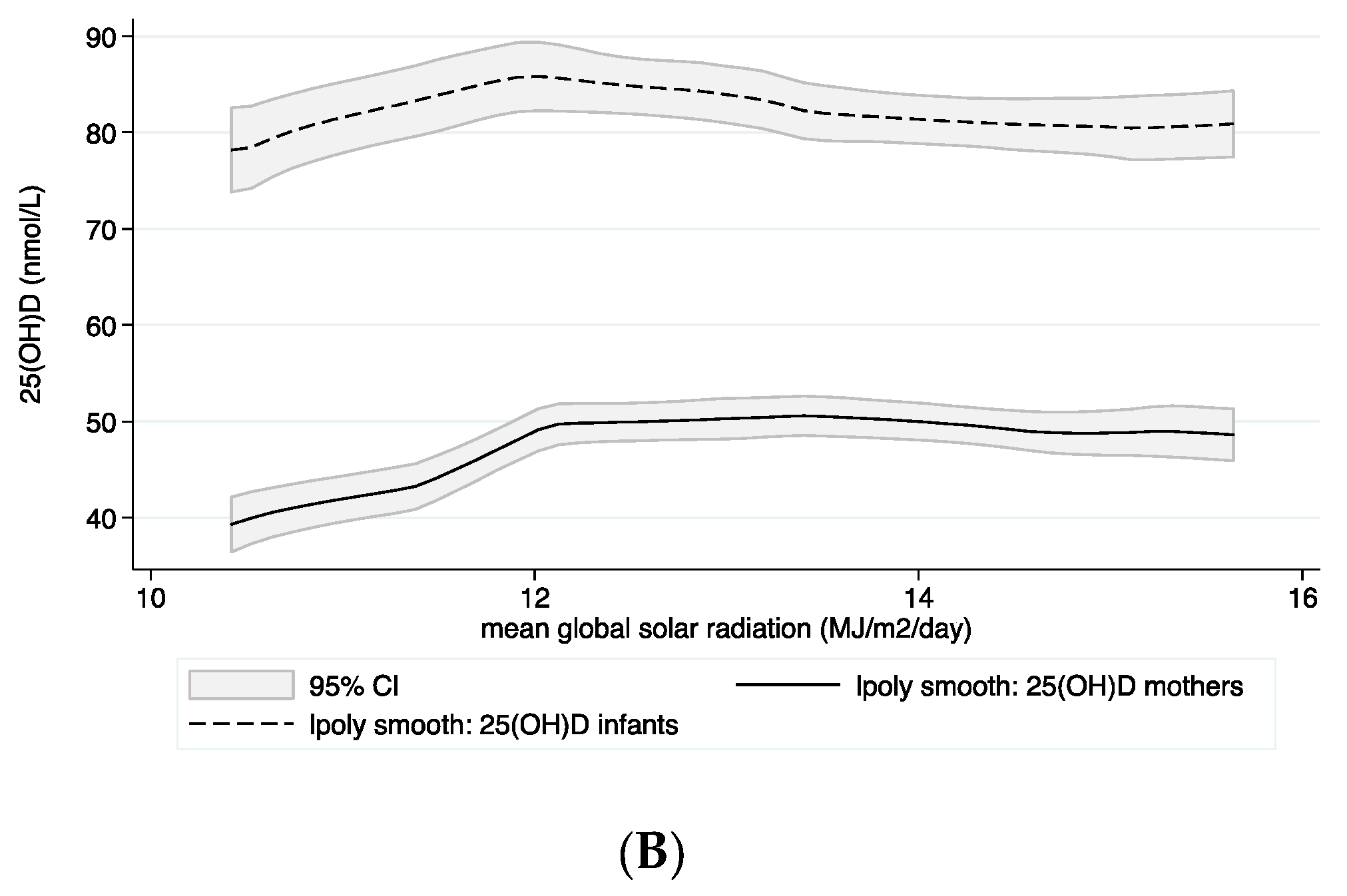
2.6. Meteorological Characteristics of the Study Area and Estimation for UVB Effect
2.7. Statistics
3. Results
3.1. Baseline Infant and Mother Characteristics
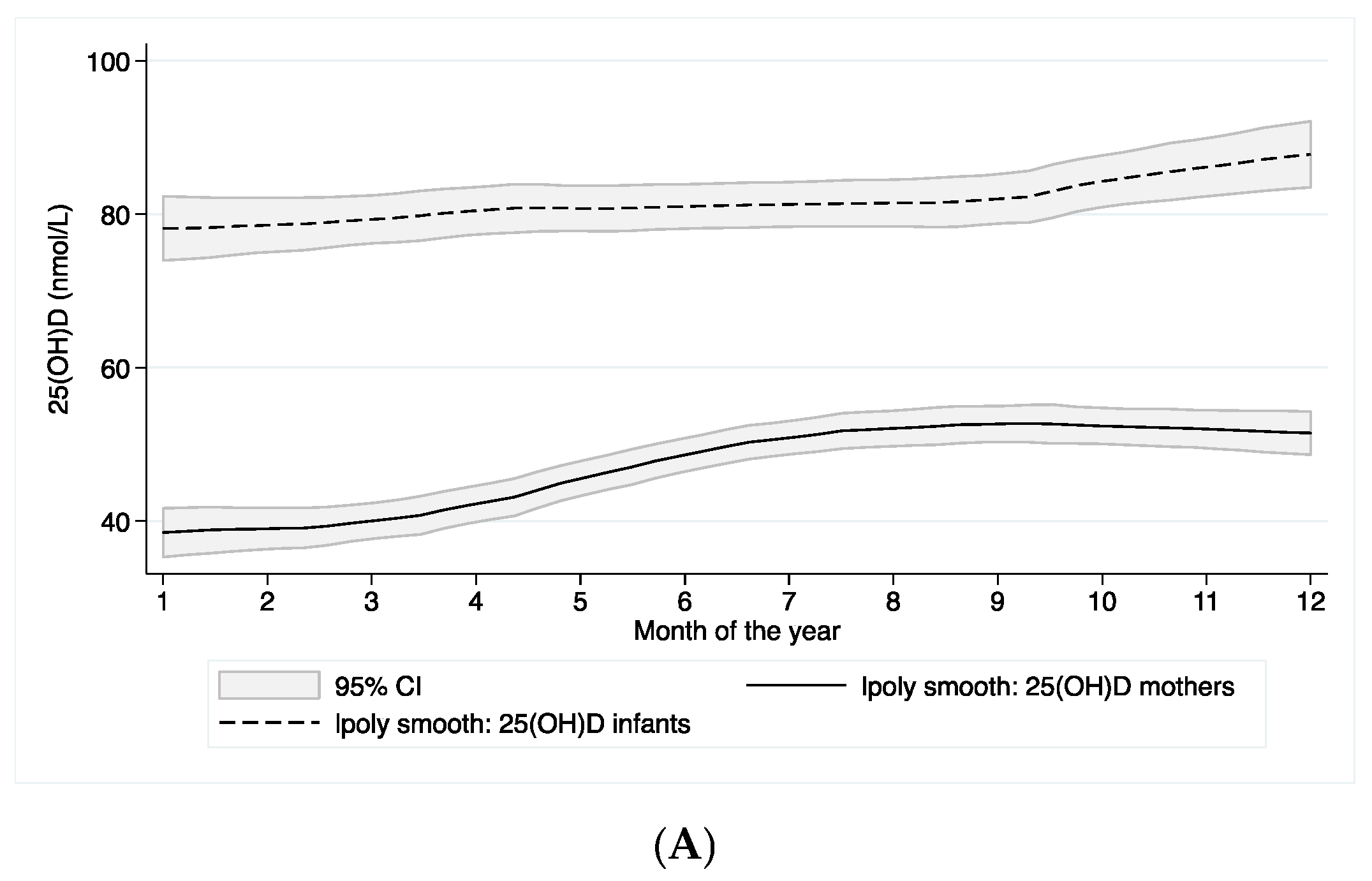
3.2. Vitamin D Status
3.3. Predictors for Plasma 25(OH)D Concentrations in Infants and Mothers
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agarwal, N.; Faridi, M.M.; Aggarwal, A.; Singh, O. Vitamin D Status of term exclusively breastfed infants and their mothers from India. Acta Paediatr. 2010, 99, 1671–1674. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, A.; Agarwal, M.; Hossain, M.; Kochiyil, J.; Zayed, R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: A justification for vitamin D supplementation of breast-feeding infants. J. Pediatr. 2003, 142, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, U.; Desai, M.; Parekh, P.; Mokal, R.; Chheda, B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007, 44, 897–901. [Google Scholar] [PubMed]
- Sachan, A.; Gupta, R.; Das, V.; Agarwal, A.; Awasthi, P.K.; Bhatia, V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am. J. Clin. Nutr. 2005, 81, 1060–1064. [Google Scholar] [PubMed]
- Hossain, N.; Khanani, R.; Hussain-Kanani, F.; Shah, T.; Arif, S.; Pal, L. High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int. J. Gynaecol. Obstet. 2011, 112, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, D.; Neupane, S.P.; Gundersen, T.E.; Madar, A.A. Vitamin D status in pre-school children in rural Nepal. Public Health Nutr. 2015, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Christian, P.; Khatry, S.K.; Wu, L.; West, K.P., Jr. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J. Nutr. 2005, 135, 1106–1112. [Google Scholar] [PubMed]
- Schulze, K.J.; Christian, P.; Wu, L.S.; Arguello, M.; Cui, H.; Nanayakkara-Bind, A.; Stewart, C.P.; Khatry, S.K.; LeClerq, S.; West, K.P., Jr. Micronutrient deficiencies are common in 6- to 8-year-old children of rural Nepal, with prevalence estimates modestly affected by inflammation. J. Nutr. 2014, 144, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.R. Estimation of Global Solar Radiation for Four Selected Sites in Nepal Using Sunshine Hours, Temperature and Relative Humidity. J. Power Energy Eng. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef] [PubMed]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 75–90. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am. J. Clin. Nutr. 2004, 79, 717–726. [Google Scholar] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1752S–1758S. [Google Scholar] [PubMed]
- Hollis, B.W.; Roos, B.A.; Draper, H.H.; Lambert, P.W. Vitamin D and its metabolites in human and bovine milk. J. Nutr. 1981, 111, 1240–1248. [Google Scholar] [PubMed]
- Dawodu, A.; Tsang, R.C. Maternal vitamin D status: Effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv. Nutr. 2012, 3, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Vieth Streym, S.; Hojskov, C.S.; Moller, U.K.; Heickendorff, L.; Vestergaard, P.; Mosekilde, L.; Rejnmark, L. Vitamin D content in human breast milk: A 9-mo follow-up study. Am. J. Clin. Nutr. 2015, 103, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L.; Howard, C.R.; Ebeling, M.; Shary, J.R.; Smith, P.G.; Taylor, S.N.; Morella, K.; Lawrence, R.A.; Hulsey, T.C. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics 2015, 136, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61 (Suppl. 3), 638S–645S. [Google Scholar]
- Prentice, A. Vitamin D deficiency: A global perspective. Nutr. Rev. 2008, 66 (Suppl. 2), S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Ulak, M.C.R.; Thorne-Lyman, A.L.; Henjum, S.; Ueland, P.M.; Midttun, Ø.; Shrestha, P.S.; Fawzi, W.W.; Graybill, L.; Strand, T.A. Vitamin Status among Breastfed Infants in Bhaktapur, Nepal. Nutrients 2016, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki—Ethical Principles of Medical Research Involving Human Subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 55th WMA Assembly, Tokyo, Japan, October 2004 (Note of Clarification added).
- Henjum, S.; Manger, M.; Skeie, E.; Ulak, M.; Thorne-Lyman, A.L.; Chandyo, R.; Shrestha, P.S.; Locks, L.; Ulvik, R.J.; Fawzi, W.W.; et al. Iron deficiency is uncommon among lactating women in urban Nepal, despite a high risk of inadequate dietary iron intake. Br. J. Nutr. 2014, 112, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chandyo, R.K.; Henjum, S.; Ulak, M.; Thorne-Lyman, A.L.; Ulvik, R.J.; Shrestha, P.S.; Locks, L.; Fawzi, W.; Strand, T.A. The prevalence of anemia and iron deficiency is more common in breastfed infants than their mothers in Bhaktapur, Nepal. Eur. J. Clin. Nutr. 2015, 18, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Henjum, S.; Torheim, L.E.; Thorne-Lyman, A.L.; Chandyo, R.; Fawzi, W.W.; Shrestha, P.S.; Strand, T.A. Low dietary diversity and micronutrient adequacy among lactating women in a peri-urban area of Nepal. Public Health Nutr. 2015, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Programme of Nutrition. WHO Global Database on Child Growth and Malnutrition; Programme of Nutrition: Geneva, Switzerland, 1997. [Google Scholar]
- Agborsangaya, C.; Toriola, A.T.; Grankvist, K.; Surcel, H.M.; Holl, K.; Parkkila, S.; Tuohimaa, P.; Lukanova, A.; Lehtinen, M. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr. Cancer 2010, 62, 51–57. [Google Scholar] [CrossRef] [PubMed]
- De la Hunty, A.; Wallace, A.M.; Gibson, S.; Viljakainen, H.; Lamberg-Allardt, C.; Ashwell, M. UK Food Standards Agency Workshop Consensus Report: The choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br. J. Nutr. 2010, 104, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; Ueland, P.M. Determination of vitamins A, D and E in a small volume of human plasma by a high-throughput method based on liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Giri, D.; Krishna Murthy, V.; Adhikary, P.R. The Influence of Meteorological Conditions on PM10 Concentrations in Kathmandu Valley. Int. J. Environ. Res. 2007, 2, 49–60. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Hoogenboezem, T.; Degenhart, H.J.; de Muinck Keizer-Schrama, S.M.; Bouillon, R.; Grose, W.F.; Hackeng, W.H.; Visser, H.K. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr. Res. 1989, 25, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Ala-Houhala, M.; Koskinen, T.; Parviainen, M.T.; Visakorpi, J.K. 25-Hydroxyvitamin D and vitamin D in human milk: Effects of supplementation and season. Am. J. Clin. Nutr. 1988, 48, 1057–1060. [Google Scholar] [PubMed]
- Wall, C.R.; Stewart, A.W.; Camargo, C.A., Jr.; Scragg, R.; Mitchell, E.A.; Ekeroma, A.; Crane, J.; Milne, T.; Rowden, J.; Horst, R.; et al. Vitamin D activity of breast milk in women randomly assigned to vitamin D3 supplementation during pregnancy. Am. J. Clin. Nutr. 2016, 103, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Hollis, B.W.; Cripps, D.J.; Tsang, R.C. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J. Pediatr. 1984, 105, 431–433. [Google Scholar] [CrossRef]
- Ministry of Health and Population (MOHP) (Nepal), New ERA, and ICF International Inc. Nepal Demographic and Health Survey 2011; Ministry of Health and Population, New ERA: Kathmandu, Nepal; ICF International: Calverton, MD, USA, 2012. [Google Scholar]
- Challa, A.; Ntourntoufi, A.; Cholevas, V.; Bitsori, M.; Galanakis, E.; Andronikou, S. Breastfeeding and vitamin D status in Greece during the first 6 months of life. Eur. J. Pediatr. 2005, 164, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Waldron, J.L.; Ashby, H.L.; Cornes, M.P.; Bechervaise, J.; Razavi, C.; Thomas, O.L.; Chugh, S.; Deshpande, S.; Ford, C.; Gama, R. Vitamin D: A negative acute phase reactant. J. Clin. Pathol. 2013, 66, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Toole, B.J.; Knox, S.; Talwar, D.; Harten, J.; O’Reilly, D.S.; Blackwell, S.; Kinsella, J.; McMillan, D.C.; Wallace, A.M. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am. J. Clin. Nutr. 2011, 93, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Bang, U.C.; Novovic, S.; Andersen, A.M.; Fenger, M.; Hansen, M.B.; Jensen, J.E. Variations in serum 25-hydroxyvitamin D during acute pancreatitis: An exploratory longitudinal study. Endocr. Res. 2011, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Lucey, A.J.; Horgan, R.; Kenny, L.C.; Kiely, M. Impact of pregnancy on vitamin D status: A longitudinal study. Br. J. Nutr. 2014, 112, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- More, C.; Bhattoa, H.P.; Bettembuk, P.; Balogh, A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 106, 209–213. [Google Scholar] [CrossRef]
- Lundqvist, A.; Sandstrom, H.; Stenlund, H.; Johansson, I.; Hultdin, J. Vitamin D Status during Pregnancy: A Longitudinal Study in Swedish Women from Early Pregnancy to Seven Months Postpartum. PLoS ONE 2016, 11, e0150385. [Google Scholar] [CrossRef] [PubMed]
- Mac Laughlin, M.F.H. Aging decreases the capasity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A. Dietary guidelines for calcium and vitamin D: A new era. Pediatrics 2011, 127, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Taylor, R.L.; Reddy, G.S.; Grebe, S.K. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M.; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value | n |
|---|---|---|
| Age in months, median (IQR) | 7 (4–9) | 487 |
| Boys, n (%) | 277 (55.4) | 500 |
| Number of siblings, mean ± SD | 1.8 ± 0.9 | 498 |
| Months of known excl. breastfeeding, median (IQR) | 3 (2–5) | 486 |
| Weight kg, mean ± SD | 7.5 ± 1.3 | 479 |
| Length/height cm, mean ± SD | 66.3 ± 4.9 | 479 |
| Wasted (<−2 SD weight for length), n (%) | 9 (1.9) | 476 |
| Underweight (<−2 SD weight for age), n (%) | 26 (5.5) | 477 |
| Stunted (<−2 SD length for age), n (%) | 46 (9.7) | 475 |
| Birth weight recall/card grams, mean ± SD | 2891.5 ± 491.8 | 438 |
| C-reactive protein in mg/L, mean ± SD | 4.3 ± 10.9 | 449 |
| Currently exclusively breastfed, n (%) | 72 (14.8) | 486 |
| Characteristics | Value | n |
|---|---|---|
| Age years, mean ± SD | 25.8 ± 4.2 | 499 |
| Current regular vitamin or mineral supplement use, n (%) | 10 (2.0) | 499 |
| Folic acid supplements during pregnancy, n (%) | 115 (23.0) | 500 |
| Iron supplement in pregnancy, n (%) | 449 (89.8) | 500 |
| Calcium supplement in pregnancy, n (%) | 402 (80.4) | 500 |
| Other medication in pregnancy, n (%) | 54 (10.8) | 500 |
| Weight kg, mean ± SD | 50.9 ± 7.6 | 499 |
| Height cm, mean ± SD | 150.5 ± 5.4 | 499 |
| BMI, mean ± SD | 22.5 ± 3.1 | 499 |
| Diastolic blood pressure, mean ± SD | 75.0 ± 8.4 | 499 |
| Systolic blood pressure, mean ± SD | 111.0 ± 10.4 | 499 |
| Mother’s energy-intake/day kcal, mean ± SD | 2089.3 ± 428.8 | 466 |
| C-reactive protein mg/L, mean ± SD | 1.7 ± 6.5 | 500 |
| Mother working outside home, n (%) | 122 (26.3) | 464 |
| Father working outside home, n (%) | 431 (93.5) | 461 |
| Mother’s education 10. grade or more, n (%) | 220 (47.4) | 464 |
| Father’s education 10. grade or more, n (%) | 298 (64.1) | 465 |
| Vitamin D Status | Infants | Mothers | ||
|---|---|---|---|---|
| Value | n | Value | n | |
| Total 25(OH)D in nmol/L, mean ± SD | 82.0 ± 21.4 | 466 | 47.4 ± 16.4 | 500 |
| Detectable 25(OH)D2, n (%) | 96 (20.6) | 466 | 12 (2.4) | 500 |
| 25(OH)D2 in nmol/L, mean ± SD | 4.7 ± 12.9 | 466 | 0.4 ± 2.7 | 500 |
| Detectable 25(OH)D3, n (%) | 466 (100) | 466 | 500 (100) | 500 |
| 25(OH)D3 in nmol/L, mean ± SD | 77.3 ± 22.0 | 466 | 47.0 ± 15.5 | 500 |
| Total 25(OH)D in nmol/L, excl. breastfed, mean ± SD | 87.1 ± 19.0 | 70 | 53.0 ± 20.2 | 72 |
| 25(OH)D3 in nmol/L, excl. breastfed, mean ± SD | 86.6 ± 19.5 | 70 | 51.8 ± 16.6 | 72 |
| Total 25(OH)D in nmol/L ≥50 nmol/L, n (%) | 449 (96.4) | 466 | 201 (40.2) | 500 |
| Total 25(OH) in nmol/L <50 nmol/L, n (%) | 17 (3.6) | 466 | 299 (59.8) | 500 |
| Total 25(OH)D in nmol/L <30 nmol/L, n (%) | 3 (0.6) | 466 | 70 (14.0) | 500 |
| Total 25(OH)D in nmol/L <75 nmol/L, n (%) | 191 (41.0) | 466 | 476 (95.2) | 500 |
| Covariate | Coeff. Crude | 95% CI | p | Coeff. Adj. | 95% CI | p | β * | R2 |
|---|---|---|---|---|---|---|---|---|
| Age of child (months) | −2.0 | −2.6, −1.3 | <0.001 | −1.5 | −2.1, −0.9 | <0.001 | −0.2 | 0.22 |
| Mothers 25(OH)D (nmol/L) | 0.5 | 0.4, 0.6 | <0.001 | 0.5 | 0.4, 0.6 | <0.001 | 0.4 | |
| Mothers BMI (kg/m2) | 0.9 | 0.3, 1.5 | 0.004 | 0.6 | 0.02, 1.2 | 0.041 | 0.08 |
| Covariate | Coeff Crude | 95% CI | p | Coeff. Adj. | 95% CI | p | β * | R2 |
|---|---|---|---|---|---|---|---|---|
| Mothers BMI (kg/m2) | 0.3 | −0.1, 0.6 | 0.109 | 0.6 | 0.2, 1.0 | 0.002 | 0.1 | 0.097 |
| Global solar radiation (MJ/m2/d) | 2.6 | 1.8, 3.4 | <0.001 | 2.6 | 1.8, 3.4 | <0.001 | 0.3 | |
| Mothers age (years) | −0.6 | −0.9, −0.2 | 0.002 | −0.7 | −1.1, −0.4 | <0.001 | −0.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haugen, J.; Ulak, M.; Chandyo, R.K.; Henjum, S.; Thorne-Lyman, A.L.; Ueland, P.M.; Midtun, Ø.; Shrestha, P.S.; Strand, T.A. Low Prevalence of Vitamin D Insufficiency among Nepalese Infants Despite High Prevalence of Vitamin D Insufficiency among Their Mothers. Nutrients 2016, 8, 825. https://doi.org/10.3390/nu8120825
Haugen J, Ulak M, Chandyo RK, Henjum S, Thorne-Lyman AL, Ueland PM, Midtun Ø, Shrestha PS, Strand TA. Low Prevalence of Vitamin D Insufficiency among Nepalese Infants Despite High Prevalence of Vitamin D Insufficiency among Their Mothers. Nutrients. 2016; 8(12):825. https://doi.org/10.3390/nu8120825
Chicago/Turabian StyleHaugen, Johanne, Manjeswori Ulak, Ram K. Chandyo, Sigrun Henjum, Andrew L. Thorne-Lyman, Per Magne Ueland, Øivind Midtun, Prakash S. Shrestha, and Tor A. Strand. 2016. "Low Prevalence of Vitamin D Insufficiency among Nepalese Infants Despite High Prevalence of Vitamin D Insufficiency among Their Mothers" Nutrients 8, no. 12: 825. https://doi.org/10.3390/nu8120825





