Comparative Study on Trace Element Excretions between Nonanuric and Anuric Patients Undergoing Continuous Ambulatory Peritoneal Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Demographic and Anthropometric Data
2.3. Dialysate Information
2.4. Biochemical Measurements
2.5. Sample Collection, Storage, and Determination of TEs
2.6. Statistical Analysis
3. Results
3.1. Study Population
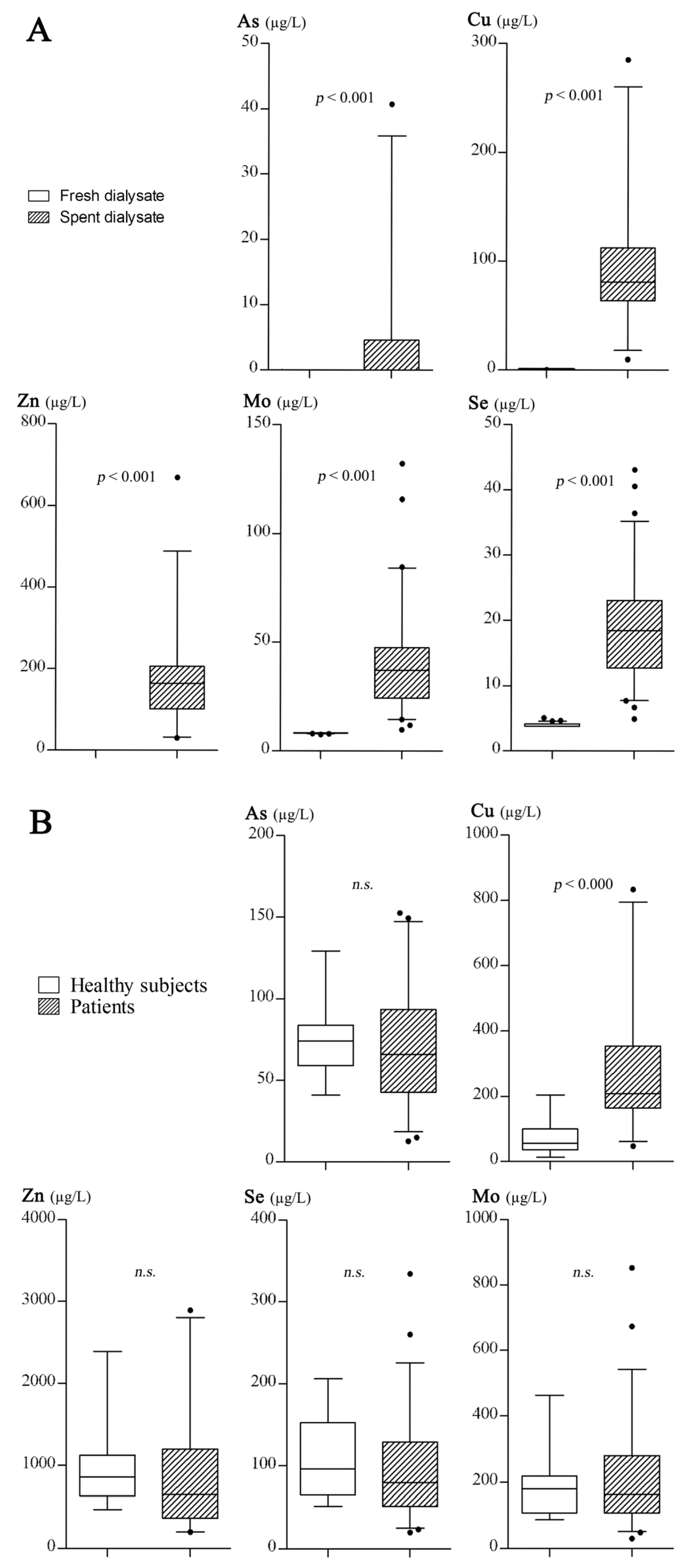
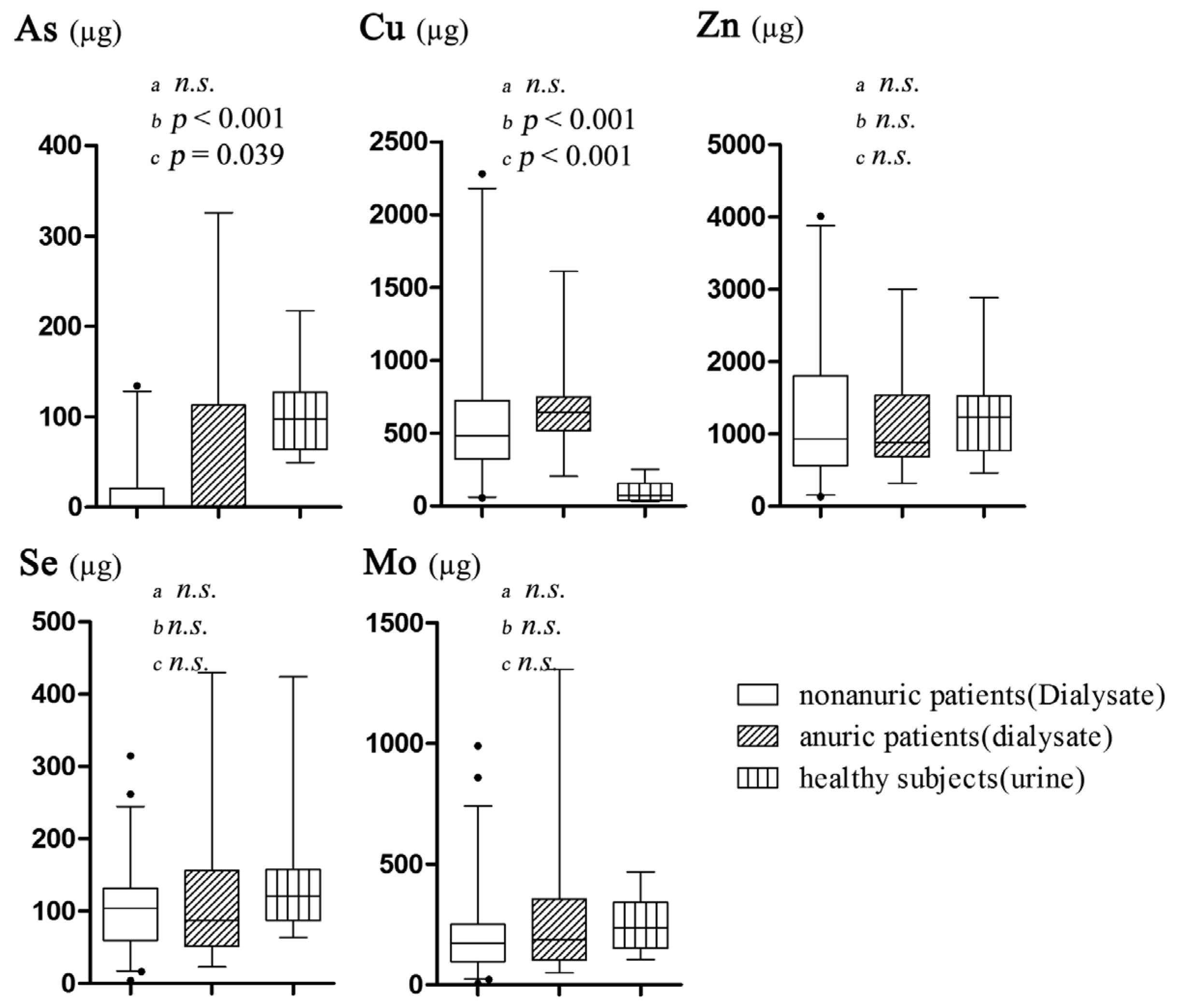
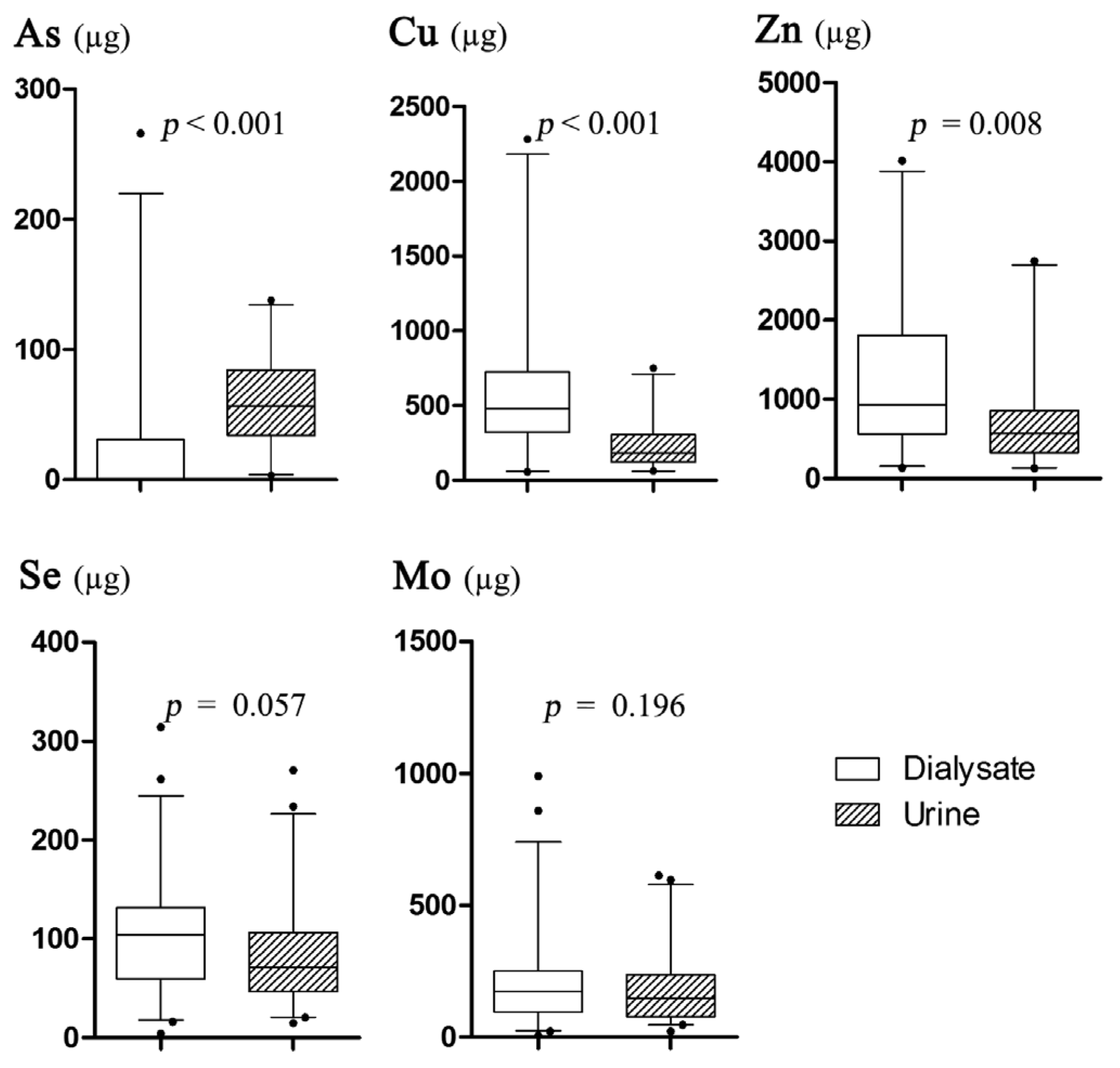
3.2. The Results of Trace Element Excretions
3.3. Correlation Analysis
4. Discussion
4.1. Characteristics of Daily Trace Element Excretions
4.2. Association between Se and Mo Excretion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| LOQ | LOQ/2 | RSD | |
|---|---|---|---|
| Cu | 0.2336 | 0.1168 | 0.8 |
| Zn | 0.0010 | 0.0005 | 1.0 |
| As | 0.0128 | 0.0064 | 0.9 |
| Se | 0.1237 | 0.0618 | 0.8 |
| Mo | 0.0036 | 0.0018 | 0.7 |
References
- Nugent, R.A.; Fathima, S.F.; Feigl, A.B.; Chyung, D. The burden of chronic kidney disease on developing nations: A 21st century challenge in global health. Nephron Clin. Pract. 2011, 118, c269–c277. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, W.; Huang, Y.; Shen, P.; Pei, D.; Yu, H.; Shi, H.; Zhang, Q.; Xu, J.; Lv, Y.; et al. Community-based study on CKD subjects and the associated risk factors. Nephrol. Dial. Transplant. 2009, 24, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, J.; Jiang, W.; Shi, Y.; He, X.; Zhou, Z.; Li, Y.; Yeung, R.O.; Wang, J.; Matsushita, K.; et al. Trends in chronic kidney disease in China. N. Engl. J. Med. 2016, 375, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef]
- Palmer, S.C.; Hayen, A.; Macaskill, P.; Pellegrini, F.; Craig, J.C.; Elder, G.J.; Strippoli, G.F. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 2011, 305, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Han, D.S. Nutrition in patients on peritoneal dialysis. Nat. Rev. Nephrol. 2012, 8, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, R.F.; Mrayyan, M.T. Assessing the prevalence of malnutrition in chronic kidney disease patients in Jordan. J. Ren. Nutr. 2008, 18, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kasama, R.K. Trace minerals in patients with end-stage renal disease. Semin. Dial. 2010, 23, 561–570. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, P.C.; Couttenye, M.M.; Lamberts, L.V.; Elseviers, M.M.; Goodman, W.G.; Schrooten, I.; Cabrera, W.E.; de Broe, M.E. Aluminum, iron, lead, cadmium, copper, zinc, chromium, magnesium, strontium, and calcium content in bone of end-stage renal failure patients. Clin. Chem. 1999, 45, 1548–1556. [Google Scholar] [PubMed]
- Morris-Stiff, G.J.; Oleesky, D.; Jurewicz, W.A. Is selenium deficiency an important risk factor for chronic graft nephropathy? A Pilot Study. Transplantation 2003, 76, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Ohsawa, M.; Itai, K.; Kato, K.; Tanno, K.; Turin, T.C.; Onoda, T.; Endo, S.; Okayama, A.; Fujioka, T. Serum selenium levels are inversely associated with death risk among hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Ari, E.; Kaya, Y.; Demir, H.; Asicioglu, E.; Keskin, S. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol. Trace Elem. Res. 2011, 144, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Selgas, R.; Romero, S.; Diez, J.J. Selenium and kidney disease. J. Nephrol. 2013, 26, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem. 2015, 68, 131–151. [Google Scholar] [PubMed]
- Rucker, D.; Thadhani, R.; Tonelli, M. Trace element status in hemodialysis patients. Semin. Dial. 2010, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hasanato, R.M. Assessment of trace elements in sera of patients undergoing renal dialysis. Saudi Med. J. 2014, 35, 365–370. [Google Scholar] [PubMed]
- Malgorzewicz, S.; Jankowska, M.; Kaczkan, M.; Czajka, B.; Rutkowski, B. Vitamins and microelements in patients with chronic kidney disease. Wiad. Lek. 2014, 67, 33–38. [Google Scholar] [PubMed]
- Krachler, M.; Scharfetter, H.; Wirnsberger, G.H. Kinetics of the metal cations magnesium, calcium, copper, zinc, strontium, barium, and lead in chronic hemodialysis patients. Clin. Nephrol. 2000, 54, 35–44. [Google Scholar] [PubMed]
- Guo, C.H.; Chen, P.C.; Hsu, G.S.; Wang, C.L. Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: A pilot study. Nutrients 2013, 5, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Sohrabi, Z.; Ekramzadeh, M.; Fallahzadeh, M.K.; Ayatollahi, M.; Geramizadeh, B.; Hassanzadeh, J.; Sagheb, M.M. Selenium supplementation improves the nutritional status of hemodialysis patients: A randomized, double-blind, placebo-controlled trial. Nephrol. Dial. Transplant. 2013, 28, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J.; The Alberta Kidney Disease Network. Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med. 2009, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esfahani, S.T.; Hamidian, M.R.; Madani, A.; Ataei, N.; Mohseni, P.; Roudbari, M.; Haddadi, M. Serum zinc and copper levels in children with chronic renal failure. Pediatr. Nephrol. 2006, 21, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Reyes-Pérez, A.; Lopez-Garcia, H.; Palomares-Bayo, M.; Olalla-Herrera, M.; Lopez-Martinez, M.C. Longitudinal study of serum zinc and copper levels in hemodialysis patients and their relation to biochemical markers. Biol. Trace Elem. Res. 2006, 113, 209–222. [Google Scholar] [CrossRef]
- Krachler, M.; Wirnsberger, G.H. Long-term changes of plasma trace element concentrations in chronic hemodialysis patients. Blood Purif. 2000, 18, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Huang, J.W.; Hung, K.Y.; Leu, L.J.; Kan, Y.T.; Yang, C.S.; Chung Wu, D.; Huang, C.L.; Chen, P.Y.; Chen, J.S.; et al. Trace metals’ abnormalities in hemodialysis patients: Relationship with medications. Artif. Organs 2000, 24, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Dlugaszek, M.; Szopa, M.; Rzeszotarski, J.; Karbowiak, P. Magnesium, calcium and trace elements distribution in serum, erythrocytes, and hair of patients with chronic renal failure. Magnes. Res. 2008, 21, 109–117. [Google Scholar] [PubMed]
- Penne, E.L.; van der Weerd, N.C.; Grooteman, M.P.; Mazairac, A.H.; van den Dorpel, M.A.; Nubé, M.J.; Bots, M.L.; Lévesque, R.; ter Wee, P.M.; Blankestijn, P.J. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Caballo, C.; Palomo, M.; Cases, A.; Galan, A.M.; Molina, P.; Vera, M.; Bosch, X.; Escolar, G.; Diaz-Ricart, M. NFkB in the development of endothelial activation and damage in uremia: An in vitro approach. PLoS ONE 2012, 7, e43374. [Google Scholar] [CrossRef] [PubMed]
- Teehan, B.P.; Schleifer, C.R.; Brown, J. Assessment of dialysis adequacy and nutrition by urea kinetic modeling. Perit. Dial. Int. 1994, 14, S99–S104. [Google Scholar] [PubMed]
- Young, G.A.; Kopple, J.D.; Lindholm, B.; Vonesh, E.F.; De Vecchi, A.; Scalamogna, A.; Castelnova, C.; Oreopoulos, D.G.; Anderson, G.H.; Bergstrom, J.; et al. Nutritional assessment of continuous ambulatory peritoneal dialysis patients: An international study. Am. J. Kidney Dis. 1991, 17, 462–471. [Google Scholar] [CrossRef]
- Feng, W.; Cui, X.; Liu, B.; Liu, C.; Xiao, Y.; Lu, W.; Guo, H.; He, M.; Zhang, X.; Yuan, J.; et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: A population-based study in China. PLoS ONE 2015, 10, e0123742. [Google Scholar] [CrossRef] [PubMed]
- Scancar, J.; Milacic, R.; Benedik, M.; Krizaj, I. Total metal concentrations in serum of dialysis patients and fractionation of Cu, Rb, Al, Fe and Zn in spent continuous ambulatory peritoneal dialysis fluids. Talanta 2003, 59, 355–364. [Google Scholar] [CrossRef]
- Apostolidis, N.S.; Panoussopoulos, D.G.; Stamou, K.M.; Kekis, P.B.; Paradellis, T.P.; Karydas, A.G.; Zarkadas, C.; Zirogiannis, P.N.; Manouras, A.J. Selenium metabolism in patients on continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 2002, 22, 400–404. [Google Scholar] [PubMed]
- Thomson, N.M. Comparison of trace elements in peritoneal dialysis, hemodialysis, and uremia. Kidney Int. 1983, 23, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Mestek, O.; Nemecek, K.; Bartova, V.; Fialova, J.; Tesar, V.; Suchanek, M. Trace elements in hemodialysis and continuous ambulatory peritoneal dialysis patients. Blood Purif. 1998, 16, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Wu, M.L.; Chou, Y.Y.; Li, S.Y.; Deng, J.F.; Yang, W.C.; Ng, Y.Y. Essential trace element status and clinical outcomes in long-term dialysis patients: A two-year prospective observational cohort study. Clin. Nutr. 2012, 31, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.H.; Wang, C.L.; Chen, P.C.; Yang, T.C. Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in patients undergoing either hemodialysis or peritoneal dialysis. Perit. Dial. Int. 2011, 31, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Reina de la Torre, M.L.; Navarro-Alarcon, M.; del Moral, L.M.; Lopez, G.S.H.; Palomares-Bayo, M.; Oliveras Lopez, M.J.; Blanca Herrera, R.M.; Agil, A. Serum Zn levels and Cu/Zn ratios worsen in hemodialysis patients, implying increased cardiovascular risk: A 2-year longitudinal study. Biol. Trace Elem. Res. 2014, 158, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, M. Biomarkers of trace mineral intake and status. J. Nutr. 2003, 133, 948S–955S. [Google Scholar] [PubMed]
- Szpanowska-Wohn, A.; Kolarzyk, E.; Chowaniec, E. Estimation of intake of zinc, copper and iron in the diet of patients with chronic renal failure treated by haemodialysis. Biol. Trace Elem. Res. 2008, 124, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Niel, O.; Thouret, M.C.; Berard, E. Anemia in congenital nephrotic syndrome: Role of urinary copper and ceruloplasmin loss. Blood 2011, 117, 6054–6055. [Google Scholar] [CrossRef] [PubMed]
- Martin-del-Campo, F.; Batis-Ruvalcaba, C.; Gonzalez-Espinoza, L.; Rojas-Campos, E.; Angel, J.R.; Ruiz, N.; Gonzalez, J.; Pazarin, L.; Cueto-Manzano, A.M. Dietary micronutrient intake in peritoneal dialysis patients: Relationship with nutrition and inflammation status. Perit. Dial. Int. 2012, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Milly, K.; Wit, L.; Diskin, C.; Tulley, R. Selenium in renal failure patients. Nephron 1992, 61, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A.; Trafikowska, U.; Adamowicz, A.; Nartowicz, E.; Manitius, J. Selenium, glutathione peroxidases, and some other antioxidant parameters in blood of patients with chronic renal failure. J. Trace Elem. Med. Biol. 2001, 15, 161–166. [Google Scholar] [CrossRef]
- Adamowicz, A.; Trafikowska, U.; Trafikowska, A.; Zachara, B.; Manitius, J. Effect of erythropoietin therapy and selenium supplementation on selected antioxidant parameters in blood of uremic patients on long-term hemodialysis. Med. Sci. Monit. 2002, 8, CR202–CR205. [Google Scholar] [PubMed]
- Chen, B.; Lamberts, L.V.; Behets, G.J.; Zhao, T.; Zhou, M.; Liu, G.; Hou, X.; Guan, G.; D’Haese, P.C. Selenium, lead, and cadmium levels in renal failure patients in China. Biol. Trace Elem. Res. 2009, 131, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marti del Moral, L.; Agil, A.; Navarro-Alarcon, M.; Lopez-Ga de la Serrana, H.; Palomares-Bayo, M.; Oliveras-Lopez, M.J. Altered serum selenium and uric acid levels and dyslipidemia in hemodialysis patients could be associated with enhanced cardiovascular risk. Biol. Trace Elem. Res. 2011, 144, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Malekmakan, L.; Hasheminasab, M. Diminished selenium levels in hemodialysis and continuous ambulatory peritoneal dialysis patients. Biol. Trace Elem. Res. 2010, 137, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Di Stasio, E.; Viola, A.; Leo, A.; Carlomagno, G.; Monteburini, T.; Cenerelli, S.; Santarelli, S.; Boggi, R.; Miggiano, G.; et al. Dietary intake of trace elements, minerals, and vitamins of patients on chronic hemodialysis. Int. Urol. Nephrol. 2014, 46, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.A.; van Lieshout, L.E.; van den Heuvel, E.G.; Matthys, C.; Peter, S.; de Groot, L.C. Conventional foods, followed by dietary supplements and fortified foods, are the key sources of vitamin D, vitamin B6, and selenium intake in Dutch participants of the nu-age study. Nutr. Res. 2016, 36, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Nhien, N.V.; Khan, N.C.; Yabutani, T.; Ninh, N.X.; Chung le, T.K.; Motonaka, J.; Nakaya, Y. Relationship of low serum selenium to anemia among primary school children living in rural Vietnam. J. Nutr. Sci. Vitaminol. 2008, 54, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tsongas, T.A.; Meglen, R.R.; Walravens, P.A.; Chappell, W.R. Molybdenum in the diet: An estimate of average daily intake in the United States. Am. J. Clin. Nutr. 1980, 33, 1103–1107. [Google Scholar] [PubMed]
- Zhang, X.; Cornelis, R.; de Kimpe, J.; Mees, L.; Lameire, N. Speciation of arsenic in serum, urine, and dialysate of patients on continuous ambulatory peritoneal dialysis. Clin. Chem. 1997, 43, 406–408. [Google Scholar] [PubMed]
- Schneidmiller, R.; Hornbostel, M.D.; Johnson, D.C. Kinetics of formation of molybdenum selenides from modulated reactants and structure of the new compound Mo3Se. Inorg. Chem. 1997, 36, 5894–5899. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Schneberg, K.A.; Pilon-Smits, E.A. Sulfur-selenium-molybdenum interactions distinguish selenium hyperaccumulator stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 2014, 239, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O.; Gremer, L.; Ferner, R.; Ferner, M.; Dobbek, H.; Gnida, M.; Meyer-Klaucke, W.; Huber, R. The role of Se, Mo and Fe in the structure and function of carbon monoxide dehydrogenase. Biol. Chem. 2000, 381, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Peng, R.D.; Jones, M.R.; Francesconi, K.A.; Goessler, W.; Howard, B.V.; Umans, J.G.; Best, L.G.; Guallar, E.; Post, W.S.; et al. Metal mixtures in urban and rural populations in the US: The multi-ethnic study of atherosclerosis and the strong heart study. Environ. Res. 2016, 147, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Maehira, F.; Luyo, G.A.; Miyagi, I.; Oshiro, M.; Yamane, N.; Kuba, M.; Nakazato, Y. Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin. Chim. Acta 2002, 316, 137–146. [Google Scholar] [CrossRef]
- Iglesias, S.B.; Leite, H.P.; Paes, A.T.; Oliveira, S.V.; Sarni, R.O. Low plasma selenium concentrations in critically ill children: The interaction effect between inflammation and selenium deficiency. Crit. Care 2014, 18, R101. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wu, P.; Hu, R.; Huang, Z. Environmental Se-Mo-B deficiency and its possible effects on crops and Keshan-Beck Disease (KBD) in the Chousang area, Yao County, Shaanxi Province, China. Environ. Geochem. Health 2003, 25, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gladyshev, V.N. Molybdoproteomes and evolution of molybdenum utilization. J. Mol. Biol. 2008, 379, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Biermann, M.; Linnemann, J.; Knupfer, U.; Vollstadt, S.; Bardl, B.; Seidel, G.; Horn, U. Trace element associated reduction of norleucine and norvaline accumulation during oxygen limitation in a recombinant Escherichia coli fermentation. Microb. Cell Fact. 2013, 12, 116. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Age (years) | 43.31 ± 12.67 | 45.00 | 19 | 68 |
| Sex (Male/Female) | 32/29 | |||
| Body height (cm) | 163.14 ± 7.66 | 163.00 | 143.00 | 176.00 |
| Body weight (kg) | 57.55 ± 10.06 | 58.00 | 36.00 | 84.70 |
| BMI (kg/m2) | 21.60 ± 3.20 | 21.20 | 14.40 | 30.80 |
| Duration of uremia (month) | 20.03 ± 26.19 | 14.00 | 1.00 | 140.00 |
| Time of dialysis (months) | 12.52 ± 13.43 | 8.00 | 3.00 | 56.00 |
| Inflow of dialysate (L) | 6.75 ± 1.22 | 6.00 | 4.00 | 8.00 |
| UF (mL/24 h) | 215.33 ± 516.32 | 175.00 | −9.00 | 1600 |
| Ccr (L/w/1.73 (m2) | 66.90 ± 19.87 | 64.94 | 33.31 | 130.14 |
| Kt/V | 2.00 ± 0.41 | 1.94 | 1.20 | 2.80 |
| nPCR | 1.10 ± 0.25 | 1.06 | 0.67 | 1.82 |
| nPNA | 0.90 ± 0.18 | 0.88 | 0.60 | 1.30 |
| Salb (g/L) | 39.41 ± 4.18 | 39.20 | 31.30 | 50.40 |
| hs-CRP (mg/L) | 3.33 ± 5.26 | 1.30 | 0.10 | 30.50 |
| FBG (mmol/L) | 5.75 ± 1.66 | 5.35 | 4.40 | 15.63 |
| eGFR | 5.31 ± 2.20 | 4.70 | 2.80 | 14.80 |
| Total protein lost in dialysate (g/day) | 4.89 ± 1.97 | 4.65 | 1.58 | 12.00 |
| Dalb (g/L) | 3.58 ± 1.36 | 3.34 | 1.08 | 7.66 |
| Dcre (μmol/L) | 680.20 ± 261.31 | 667.00 | 4.50 | 1330.00 |
| Dun (mmol/L) | 15.96 ± 4.68 | 15.84 | 5.54 | 28.04 |
| Upro (g/L) | 0.60 ± 0.53 | 0.42 | 0.01 | 2.22 |
| Ualb (g/L) | 0.36 ± 0.35 | 0.29 | 0.01 | 1.73 |
| UUA (μmol/L) | 460.83 ± 254.20 | 416.50 | 2.17 | 1153.00 |
| Ucre (μmol/L) | 5234.81 ± 2994.75 | 4424.50 | 257.00 | 12,112.00 |
| Uun (mmol/L) | 57.96 ± 33.21 | 53.00 | 20.00 | 202.00 |
| Patients | Healthy Subjects (n = 11) | |||
|---|---|---|---|---|
| All (n = 61) | Nonanuric Patients (n = 45) | Anuric Patients (n = 16) | ||
| Age (years) | 43.31 a ± 12.67 | 43.39 ± 11.11 | 43.38 ± 16.73 | 29.82 ± 7.82 |
| Sex (Male/Female) | 32/29 | 20/25 | 11/5 | 6/5 |
| BMI (kg/m2) | 21.60 ± 3.20 | 21.20 ± 2.99 | 22.79 ± 3.57 | 21.06 ± 2.39 |
| Time of dialysis (months) | 12.52 (3.00–56.00) | 5.00 d (3.00–51.00) | 16.50(3.00–56.00) | - |
| Cu (μg/day) | 672.49 a | 771.01 b | 653.54 c | 72.76 |
| (205.40–2447.69) | (244.23–2447.69) | (205.40–1610.92) | (29.4–251.17) | |
| Zn (μg/day) | 1546.14 | 1757.65 b,d | 1099.30 | 1232.52 |
| (321.97–4622.47) | (830.30–4280.55) | (351.97–4622.47) | (463.34–2889.00) | |
| Se (μg/day) | 158.76 | 173.04 b,d | 98.19 | 120.79 |
| (23.26–548.35) | (94.41–548.35) | (23.26–429.86) | (63.32–423.55) | |
| Mo (μg/day) | 309.49 | 319.45 b,d | 219.03 | 237.20 |
| (49.41–1586.19) | (143.09–1586.19) | (49.41–1307.71) | (105.16–467.84) | |
| As (μg/day) | 69.44 | 70.67 d | 4.8 c | 97.81 |
| (0.01–325.75) | (11.21–169.44) | (0.01–325.75) | (49.61–217.39) | |
| Cu | Zn | Se | Mo | As | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β1 | β2 | β1 | β2 | β1 | β2 | β1 | β2 | β1 | β2 | |
| Cu | 1 | 1 | ||||||||
| Zn | 0.182 | 0.458 ** | 1 | 1 | ||||||
| Se | 0.483 ** | 0.394 ** | −0.011 | 0.684 ** | 1 | 1 | ||||
| Mo | 0.327 * | 0.346 * | −0.071 | 0.607 * | 0.869 ** | 0.968 ** | 1 | 1 | ||
| As | 0.121 | 0.396 ** | 0.023 | 0.510 ** | 0.027 | 0.688 ** | 0.071 | 0.662 ** | 1 | 1 |
| Cu | Zn | Se | Mo | |
|---|---|---|---|---|
| Salb (g/L) | 0.034 | 0.039 | 0.201 | 0.203 |
| hs-CRP (mg/L) | −0.025 | −0.012 | −0.417 ** | −0.431 ** |
| FGB (mmol/L) | 0.086 | −0.035 | −0.074 | 0.057 |
| eGFR (mL/min/1.73 m2) | −0.097 | −0.205 | −0.022 | −0.174 |
| Dpro (g/day) | 0.588 ** | −0.141 | 0.394 ** | 0.314 * |
| Dalb (g/L) | 0.614 ** | 0.214 | 0.434 ** | 0.338 ** |
| Dcre (umol/L) | 0.369 ** | −0.057 | 0.333 ** | 0.347 ** |
| Dun (mmol/L) | −0.083 | −0.208 | 0.061 | 0.070 |
| nPNA | −0.169 | −0.129 | −0.021 | −0.022 |
| nPCR | −0.243 | −0.171 | −0.060 | −0.088 |
| Cu | Zn | Se | Mo | |
|---|---|---|---|---|
| Salb (g/L) | −0.140 | 0.105 | 0.242 | 0.270 |
| Sua (umol/L) | 0.045 | 0.383 ** | 0.208 | 0.249 |
| FGB (mmol/L) | −0.172 | 0.007 | −0.033 | −0.019 |
| eGFR | −0.262 | 0.370 * | 0.368 * | 0.363 * |
| Upro (g/L) | 0.455 ** | −0.212 | 0.353 * | 0.382 ** |
| Ualb (g/L) | 0.456 ** | 0.263 | 0.374 ** | 0.413 ** |
| Uua (umol/L) | 0.332 * | 0.524 ** | 0.496 ** | 0.486 ** |
| Ucre (umol/L) | 0.014 | 0.466 ** | 0.329 * | 0.353 * |
| Uun (mmol/L) | 0.236 | 0.538 ** | 0.580 ** | 0.604 ** |
| nPNA | 0.320 * | 0.046 | 0.225 | 0.223 |
| nPCR | 0.367 ** | 0.340 * | 0.408 ** | 0.413 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, S.; Yao, Y.; Wan, Y.; Liang, W.; Meng, R.; Jin, Q.; Wu, N.; Xu, F.; Ying, C.; Zuo, X. Comparative Study on Trace Element Excretions between Nonanuric and Anuric Patients Undergoing Continuous Ambulatory Peritoneal Dialysis. Nutrients 2016, 8, 826. https://doi.org/10.3390/nu8120826
Xiang S, Yao Y, Wan Y, Liang W, Meng R, Jin Q, Wu N, Xu F, Ying C, Zuo X. Comparative Study on Trace Element Excretions between Nonanuric and Anuric Patients Undergoing Continuous Ambulatory Peritoneal Dialysis. Nutrients. 2016; 8(12):826. https://doi.org/10.3390/nu8120826
Chicago/Turabian StyleXiang, Siyun, Ying Yao, Yunan Wan, Wangqun Liang, Ruiwei Meng, Qiman Jin, Nannan Wu, Fangyi Xu, Chenjiang Ying, and Xuezhi Zuo. 2016. "Comparative Study on Trace Element Excretions between Nonanuric and Anuric Patients Undergoing Continuous Ambulatory Peritoneal Dialysis" Nutrients 8, no. 12: 826. https://doi.org/10.3390/nu8120826





