Antioxidants and Dementia Risk: Consideration through a Cerebrovascular Perspective
Abstract
:1. Introduction
2. Cerebrovascular Integrity in Neurodegeneration, Cognitive Decline and Dementia
2.1. Dysfunction of the Cerebrovascular Blood-Brain Barrier
2.2. Blood-Brain Barrier Dysfunction in Cognitive Deficits and Dementia
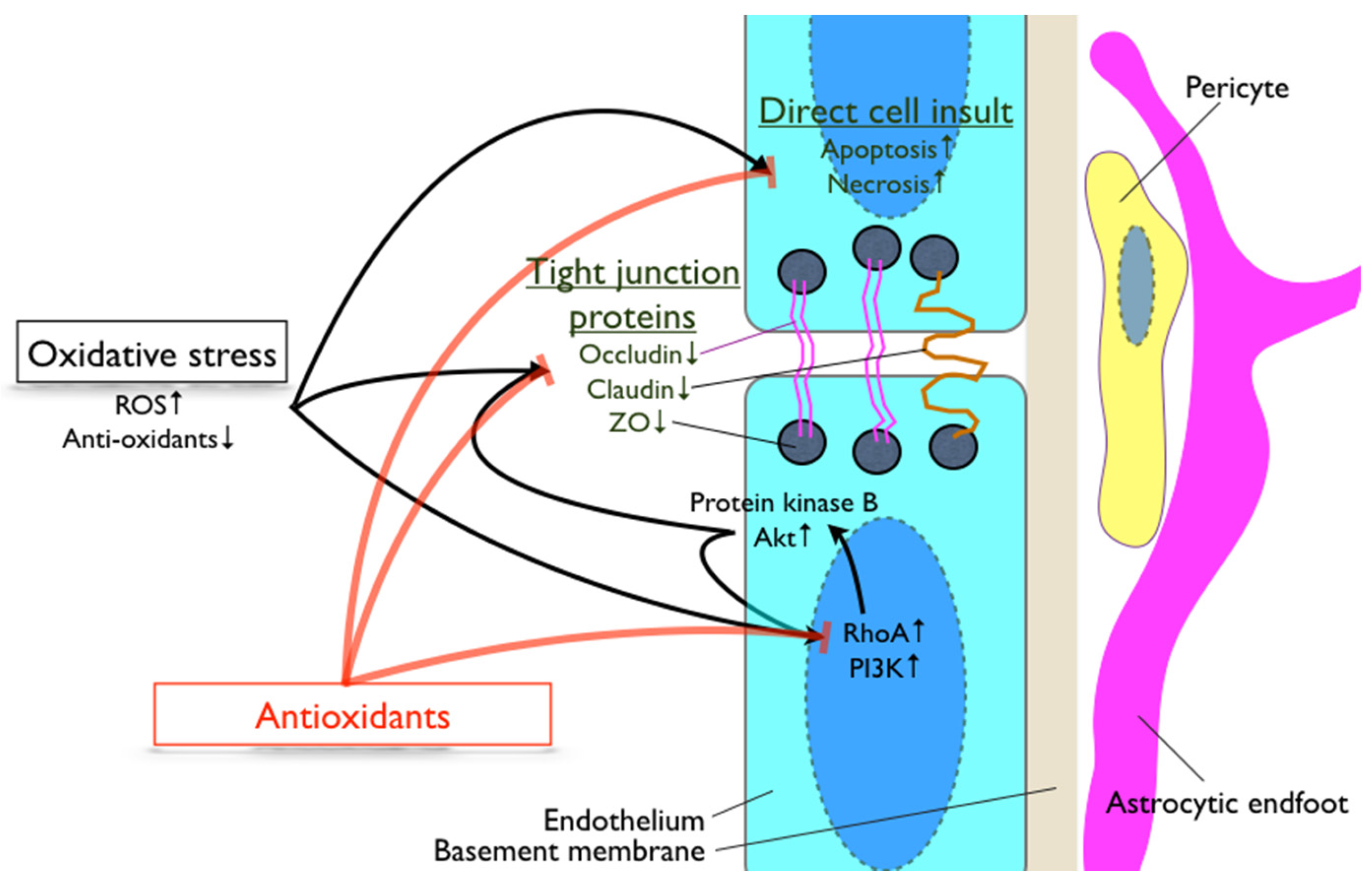
3. Involvement of Oxidative Stress during Breakdown of the Blood-Brain Barrier
4. Antioxidants, Cognitive Decline and Blood-Brain Barrier
4.1. Anti-Oxidative Vitamins
4.2. Other Natural Antioxidants
4.3. Lipid-Lowering Drugs with Anti-Oxidative Properties
4.4. Other Pharmacological Agents with Anti-Oxidative Effects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Organization, W.H. Dementia a Public Health Priority; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jonsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2016. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.A.; Lochner, K.A.; Thambisetty, M.; Wingo, T.S.; Posner, S.F.; Ling, S.M. Prevalence of dementia subtypes in US Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M. The blood-brain barrier. Neurobiol. Dis. 2010, 37, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in repositioning Alzheimer’s disease drugs based on a multitarget strategy. Future Med. Chem. 2016, 8, 2113–2142. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.; Gilbey, P.; Asli, H.; Khamisy-Farah, R.; Assy, N. Antioxidant enzyme activity and cognition in obese individuals with or without metabolic risk factors. Exp. Clin. Endocrinol. Diabetes 2016, 124, 568–571. [Google Scholar] [PubMed]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.M.; Lam, V.; Mamo, J.C. Dietary fats, cerebrovasculature integrity and Alzheimer’s disease risk. Prog. Lipid Res. 2010, 49, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.M.; Wellington, C.L.; Johnsen, R.D.; Dhaliwal, S.S.; Mamo, J.C. Differential effects of dietary fatty acids on the cerebral distribution of plasma-derived apo B lipoproteins with amyloid-β. Br. J. Nutr. 2010, 103, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Vascular basis for brain degeneration: Faltering controls and risk factors for dementia. Nutr. Rev. 2010, 68 (Suppl. 2), S74–S87. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Giordano, J.; Signorile, A.; Laura Ontario, M.; Castorina, S.; De Pasquale, C.; Eckert, G.; Calabrese, E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016, 94, 1588–1603. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Soejitno, A.; Tjan, A.; Purwata, T.E. Alzheimer’s disease: Lessons learned from amyloidocentric clinical trials. CNS Drugs 2015, 29, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.M.; Mamo, J.C. Chylomicron amyloid-beta in the aetiology of Alzheimer’s disease. Atheroscler. Suppl. 2008, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Vinters, H.V.; Cole, G.M.; Khachaturian, Z.S. Alzheimer’s disease: Etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 1998, 51, S2–S17. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. Rage mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Candela, P.; Gosselet, F.; Saint-Pol, J.; Sevin, E.; Boucau, M.C.; Boulanger, E.; Cecchelli, R.; Fenart, L. Apical-to-basolateral transport of amyloid-β peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J. Alzheimers Dis. 2010, 22, 849–859. [Google Scholar] [PubMed]
- Hultman, K.; Strickland, S.; Norris, E.H. The apoe ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J. Cereb. Blood Flow Metab. 2013, 33, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Kaye, J.A.; Quinn, J.F. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr. Gerontol. Geriatr. Res. 2012, 2012, 184042. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.; Wellington, C.; Johnsen, R.; Mamo, J.C. Three-dimensional colocalization analysis of plasma-derived apolipoprotein B with amyloid plaques in APP/PS1 transgenic mice. Histochem. Cell Biol. 2009, 131, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nation, D.A.; Pa, J.; Sweeney, M.D.; Toga, A.W.; Zlokovic, B.V. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016, 131, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Van de Haar, H.J.; Burgmans, S.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Hofman, P.A.; Verhey, F.R.; Backes, W.H. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 2016, 281, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Dickstein, D.L.; Carlow, D.A.; Jefferies, W.A. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 2003, 10, 463–470. [Google Scholar] [PubMed]
- Pun, P.B.; Lu, J.; Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; McCaffrey, G.; Quigley, C.E.; Finch, J.; DeMarco, K.M.; Nametz, N.; Davis, T.P. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J. Cereb. Blood Flow Metab. 2010, 30, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Tumer, N. Oxidative stress, brain edema, blood-brain barrier permeability, and autonomic dysfunction from traumatic brain injury. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ronaldson, P.T.; Davis, T.P. Targeting transporters: Promoting blood-brain barrier repair in response to oxidative stress injury. Brain Res. 2015, 1623, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Chrissobolis, S.; Faraci, F.M. The role of oxidative stress and nadph oxidase in cerebrovascular disease. Trends Mol. Med. 2008, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- VanGilder, R.L.; Kelly, K.A.; Chua, M.D.; Ptachcinski, R.L.; Huber, J.D. Administration of sesamol improved blood-brain barrier function in streptozotocin-induced diabetic rats. Exp. Brain Res. 2009, 197, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Musters, R.J.; Reijerkerk, A.; de Groot, L.R.; van der Pol, S.M.; Hendrikx, E.M.; Dopp, E.D.; Dijkstra, C.D.; Drukarch, B.; de Vries, H.E. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J. Immunol. 2006, 177, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007, 101, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Shiu, C.; Barbier, E.; Di Cello, F.; Choi, H.J.; Stins, M. HIV-1 gp120 as well as alcohol affect blood-brain barrier permeability and stress fiber formation: Involvement of reactive oxygen species. Alcohol. Clin. Exp. Res. 2007, 31, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer’s. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Markesbery, W.R. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J. Neurosci. Res. 2007, 85, 3036–3040. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Associations between sleep, cortisol regulation, and diet: Possible implications for the risk of Alzheimer disease. Adv. Nutr. 2016, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Riviere, S.; Birlouez-Aragon, I.; Nourhashemi, F.; Vellas, B. Low plasma Vitamin C in Alzheimer patients despite an adequate diet. Int. J. Geriatr. Psychiatry 1998, 13, 749–754. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Delmas-Beauvieux, M.C.; Peuchant, E.; Richard-Harston, S.; Decamps, A.; Reignier, B.; Emeriau, J.P.; Rainfray, M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 2001, 30, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.; Breteler, M.M. Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C.; Cache County Study, G. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The cache county study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Man, Y.; Li, N.; Zhou, Y.U. Effects of Vitamins E and C combined with β-carotene on cognitive function in the elderly. Exp. Ther. Med. 2015, 9, 1489–1493. [Google Scholar] [PubMed]
- Harrison, F.E.; Hosseini, A.H.; McDonald, M.P.; May, J.M. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol. Biochem. Behav. 2009, 93, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Montilla-Lopez, P.; Munoz-Agueda, M.C.; Feijoo Lopez, M.; Munoz-Castaneda, J.R.; Bujalance-Arenas, I.; Tunez-Finana, I. Comparison of melatonin versus Vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer’s disease induced by okadaic acid in neuroblastoma cells. Eur. J. Pharmacol. 2002, 451, 237–243. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, S.; Swarnakar, S.; Das, N. Nanocapsulated ascorbic acid in combating cerebral ischemia reperfusion-induced oxidative injury in rat brain. Curr. Alzheimer Res. 2016, 13, 1363–1373. [Google Scholar]
- Takasaki, J.; Ono, K.; Yoshiike, Y.; Hirohata, M.; Ikeda, T.; Morinaga, A.; Takashima, A.; Yamada, M. Vitamin A has anti-oligomerization effects on amyloid-β in vitro. J. Alzheimers Dis. 2011, 27, 271–280. [Google Scholar] [PubMed]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of Vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Huang, Y.H.; Shen, Y.C.; Huang, H.C.; Liu, P.H. Ascorbic acid prevents blood-brain barrier disruption and sensory deficit caused by sustained compression of primary somatosensory cortex. J. Cereb. Blood Flow Metab. 2010, 30, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Allahtavakoli, M.; Amin, F.; Esmaeeli-Nadimi, A.; Shamsizadeh, A.; Kazemi-Arababadi, M.; Kennedy, D. Ascorbic acid reduces the adverse effects of delayed administration of tissue plasminogen activator in a rat stroke model. Basic Clin. Pharmacol. Toxicol. 2015, 117, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Park, J.; Kim, J.H.; Choi, J.Y.; Kim, J.Y.; Lee, K.M.; Lee, J.E. Dehydroascorbic acid attenuates ischemic brain edema and neurotoxicity in cerebral ischemia: An in vivo study. Exp. Neurobiol. 2015, 24, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.L.; Bayraktutan, U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes. Metab. 2009, 11, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; He, Q.; Tong, Y.; Zhan, R.; Xu, F.; Fan, D.; Guo, X.; Han, H.; Qin, S.; Chui, D. Phospholipid transfer protein (PLTP) deficiency impaired blood-brain barrier integrity by increasing cerebrovascular oxidative stress. Biochem. Biophys. Res. Commun. 2014, 445, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Oztas, B.; Akgul, S.; Seker, F.B. Gender difference in the influence of antioxidants on the blood-brain barrier permeability during pentylenetetrazol-induced seizures in hyperthermic rat pups. Biol. Trace Element Res. 2007, 118, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.O.; Starkey, S.R.; Stipetic, K.; Divers, T.J.; Summers, B.A.; de Lahunta, A. The role of dietary antioxidant insufficiency on the permeability of the blood-brain barrier. J. Neuropathol. Exp. Neurol. 2008, 67, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Vornicescu, C.; Bosca, B.; Crisan, D.; Yacoob, S.; Stan, N.; Filip, A.; Sovrea, A. Neuroprotective effect of melatonin in experimentally induced hypobaric hypoxia. Rom. J. Morphol. Embryol. 2013, 54, 1097–1106. [Google Scholar] [PubMed]
- Alluri, H.; Wilson, R.L.; Anasooya Shaji, C.; Wiggins-Dohlvik, K.; Patel, S.; Liu, Y.; Peng, X.; Beeram, M.R.; Davis, M.L.; Huang, J.H.; et al. Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS ONE 2016, 11, e0154427. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kang, S.M.; Lee, W.T.; Park, K.A.; Lee, K.M.; Lee, J.E. The beneficial effect of melatonin in brain endothelial cells against oxygen-glucose deprivation followed by reperfusion-induced injury. Oxid. Med. Cell. Longev. 2014, 2014, 639531. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Pallebage-Gamarallage, M.M.; Lam, V.; Giles, C.; Mamo, J.C. Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood-brain barrier function in wild-type mice. J. Neuroinflamm. 2013, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Huang, C.C.; Chio, C.C.; Tsai, K.J.; Chang, C.P.; Lin, N.K.; Lin, M.T. Inhibition of peripheral TNF-α and downregulation of microglial activation by alpha-lipoic acid and etanercept protect rat brain against ischemic stroke. Mol. Neurobiol. 2016, 53, 4961–4971. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, M.P.; Cipolla, M.J. Cerebrovascular dysfunction and blood-brain barrier permeability induced by oxidized LDL are prevented by apocynin and magnesium sulfate in female rats. J. Cardiovasc. Pharmacol. 2014, 63, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Cai, Y.B.; Liu, Z. Activation of AMPK improves lipopolysaccharide-induced dysfunction of the blood-brain barrier in mice. Brain Inj. 2015, 29, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hu, J.; Gao, X.; Liang, H.; Liu, Z. Activation of AMPK attenuates lipopolysaccharide-impaired integrity and function of blood-brain barrier in human brain microvascular endothelial cells. Exp. Mol. Pathol. 2014, 97, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lai, L.; Li, X.; Zhang, X.; He, X.; Liu, W.; Li, R.; Ke, X.; Fu, C.; Huang, Z.; et al. Baicalein attenuates neurological deficits and preserves blood-brain barrier integrity in a rat model of intracerebral hemorrhage. Neurochem. Res. 2016, 41, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, X.; Roche, I.; Liu, R.; Geiger, J.D. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J. Neurochem. 2008, 107, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gawryluk, J.W.; Wagener, J.F.; Ghribi, O.; Geiger, J.D. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J. Neuroinflamm. 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jiang, M.; Fang, J.; Yang, M.F.; Zhang, S.; Yin, Y.X.; Li, D.W.; Mao, L.L.; Fu, X.Y.; Hou, Y.J.; et al. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Mol. Neurobiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, W.; Sun, Y.J.; Hu, M.; Li, F.; Zhu, D.Y. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur. J. Pharmacol. 2007, 561, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, Z.L.; Qin, Z.H.; Liang, Z.Q. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol. Sin. 2008, 29, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, R.; Gao, M.; Wang, Y.; Yu, X.; Xuan, Z.; Sun, J.; Yang, F.; Wu, C.; Du, G. Pinocembrin attenuates blood-brain barrier injury induced by global cerebral ischemia-reperfusion in rats. Brain Res. 2011, 1391, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhu, S.Y.; Tan, C.B.; Xu, B.; Zhang, W.C.; Du, G.H. Pinocembrin protects the neurovascular unit by reducing inflammation and extracellular proteolysis in MCAO rats. J. Asian Nat. Prod. Res. 2010, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.P.; Fu, J.S.; Zhang, S.; Bai, L.; Guo, L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J. Neurophysiol. 2016, 116, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Liu, B. Resveratrol attenuates lipopolysaccharide-induced dysfunction of blood-brain barrier in endothelial cells via AMPK activation. Korean J. Physiol. Pharmacol. 2016, 20, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.F.; Li, N.; Wang, Q.; Cheng, X.J.; Li, X.M.; Liu, T.T. Resveratrol decreases the insoluble Aβ1–42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience 2015, 310, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, S.; Zhen, L.; Yang, Q.; Wu, Z.; Lei, X.; Lv, J.; Xiong, L.; Xue, R. Resveratrol attenuates the blood-brain barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after cerebral ischemia reperfusion in rats. J. Mol. Neurosci. 2015, 55, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Tai, Y.T.; Cherng, Y.G.; Lin, J.W.; Liu, S.H.; Chen, T.L.; Chen, R.M. Resveratrol attenuates high-fat diet-induced disruption of the blood-brain barrier and protects brain neurons from apoptotic insults. J. Agric. Food Chem. 2014, 62, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, J.; Feng, J. Treatment with tanshinone IIA suppresses disruption of the blood-brain barrier and reduces expression of adhesion molecules and chemokines in experimental autoimmune encephalomyelitis. Eur. J. Pharmacol. 2016, 771, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Feng, J.; Zhou, R.; Ye, L.Y.; Liu, H.L.; Peng, L.; Lou, J.N.; Li, C.H. Tanshinone IIA protects the human blood-brain barrier model from leukocyte-associated hypoxia-reoxygenation injury. Eur. J. Pharmacol. 2010, 648, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Xue, H.; Bai, C.; Fu, R.; Wu, A. The effects of tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomed. Int. J. Phytother. Phytopharmacol. 2010, 17, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Kho, D.; Graham, E.S.; Nicholson, L.F.; O’Carroll, S.J. Statins inhibit fibrillary β-amyloid induced inflammation in a model of the human blood brain barrier. PLoS ONE 2016, 11, e0157483. [Google Scholar] [CrossRef] [PubMed]
- Pallebage-Gamarallage, M.; Lam, V.; Takechi, R.; Galloway, S.; Clark, K.; Mamo, J. Restoration of dietary-fat induced blood-brain barrier dysfunction by anti-inflammatory lipid-modulating agents. Lipids Health Dis. 2012, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.M.; Lam, V.; Dhaliwal, S.S.; Mamo, J.C. Probucol prevents blood-brain barrier dysfunction in wild-type mice induced by saturated fat or cholesterol feeding. Clin. Exp. Pharmacol. Physiol. 2013, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Pallebage-Gamarallage, M.M.; Lam, V.; Giles, C.; Mamo, J.C. Long-term probucol therapy continues to suppress markers of neurovascular inflammation in a dietary induced model of cerebral capillary dysfunction. Lipids Health Dis. 2014, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, L.; Zhang, B.; Park, M.; Toborek, M. Ppar agonist-mediated protection against HIV Tat-induced cerebrovascular toxicity is enhanced in MMP-9-deficient mice. J. Cereb. Blood Flow Metab. 2014, 34, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Mysiorek, C.; Culot, M.; Dehouck, L.; Derudas, B.; Staels, B.; Bordet, R.; Cecchelli, R.; Fenart, L.; Berezowski, V. Peroxisome-proliferator-activated receptor-α activation protects brain capillary endothelial cells from oxygen-glucose deprivation-induced hyperpermeability in the blood-brain barrier. Curr. Neurovasc. Res. 2009, 6, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Reiter, R.J.; Acuna-Castroviejo, D.; Tan, D.X.; Burkhardt, S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Annu. N. Y. Acad. Sci. 2001, 939, 200–215. [Google Scholar] [CrossRef]
- Sirin, F.B.; Kumbul Doguc, D.; Vural, H.; Eren, I.; Inanli, I.; Sutcu, R.; Delibas, N. Plasma 8-isoPGF2α and serum melatonin levels in patients with minimal cognitive impairment and Alzheimer disease. Turk. J. Med. Sci. 2015, 45, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, R.M.; van Munster, B.C.; van Kempen, M.F.; de Rooij, S.E. Physiological melatonin levels in healthy older people: A systematic review. J. Psychosom. Res. 2016, 86, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Tone, N.; Tanaka, K.; Kataoka, H.; Morikawa, M.; Kurumatani, N. Physiological levels of melatonin relate to cognitive function and depressive symptoms: The HEIJO-KYO cohort. J. Clin. Endocrinol. Metab. 2015, 100, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Rudnitskaya, E.A.; Muraleva, N.A.; Maksimova, K.Y.; Kiseleva, E.; Kolosova, N.G.; Stefanova, N.A. Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J. Alzheimers Dis. 2015, 47, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Mukda, S.; Panmanee, J.; Boontem, P.; Govitrapong, P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci. Lett. 2016, 621, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhao, S.D.; Liu, H.J.; Yuan, Q.H.; Liu, S.M.; Zhang, Y.M.; Ling, E.A.; Hao, A.J. Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J. Pineal Res. 2011, 51, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sotthibundhu, A.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J. Pineal Res. 2010, 49, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Maksimova, K.Y.; Kiseleva, E.; Rudnitskaya, E.A.; Muraleva, N.A.; Kolosova, N.G. Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression of Alzheimer’s disease-like pathology. J. Pineal Res. 2015, 59, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Munch, G. α-lipoic acid as a new treatment option for Alzheimer’s disease—A 48 months follow-up analysis. J. Neural Transm. Suppl. 2007, 189–193. [Google Scholar]
- Zhao, R.R.; Xu, F.; Xu, X.C.; Tan, G.J.; Liu, L.M.; Wu, N.; Zhang, W.Z.; Liu, J.X. Effects of alpha-lipoic acid on spatial learning and memory, oxidative stress, and central cholinergic system in a rat model of vascular dementia. Neurosci. Lett. 2015, 587, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J. Alzheimers Dis. 2012, 32, 447–455. [Google Scholar] [PubMed]
- Mathew, B.; Biju, R. Neuroprotective effects of garlic: A review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. The “aged garlic extract” (AGE) and one of its active ingredients s-allyl-l-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD). Curr. Med. Chem. 2011, 18, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006, 108, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B.; Sandoval, J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother. Res. 2007, 21, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-l-cysteine treatment in the neuronal culture and APP-Tg mouse model. J. Neurochem. 2011, 117, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ghribi, O.; Geiger, J.D. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S127–S141. [Google Scholar] [PubMed]
- Lan, X.; Wang, W.; Li, Q.; Wang, J. The natural flavonoid pinocembrin: Molecular targets and potential therapeutic applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Sarkar, C.; Singh, S.P.; Zhang, Z.; Munasinghe, J.; Peng, S.; Chandra, G.; Kong, E.; Mukherjee, A.B. The blood-brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: Amelioration by resveratrol. Hum. Mol. Genet. 2012, 21, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Nie, Y.; Zhang, X.; Tan, B.; Cao, H.; Chen, W.; Gao, W.; Chen, J.; Liang, Z.; Lai, H.; et al. Effects of grape seed proanthocyanidin on Alzheimer’s disease in vitro and in vivo. Exp. Ther. Med. 2016, 12, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Parihar, M.S.; Zenebe, W.J.; Ghafourifar, P. Statins lower calcium-induced oxidative stress in isolated mitochondria. Hum. Exp. Toxicol. 2012, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Szerej, A.; Kurzepa, J.; Wojczal, J.; Stelmasiak, Z. Simvastatin displays an antioxidative effect by inhibiting an increase in the serum 8-isoprostane level in patients with acute ischemic stroke: Brief report. Clin. Neuropharmacol. 2011, 34, 191–194. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, B.; Craig, D.; Bullock, R.; Passmore, P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Mospan, C.M. Are statins protective or harmful to cognitive function? JAAPA 2016, 29, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, H.C.; Hake, A.; Lane, K.; Purnell, C.; Unverzagt, F.; Smith-Gamble, V.; Murrell, J.; Ogunniyi, A.; Baiyewu, O.; Callahan, C.; et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn. Dis. 2015, 25, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Schumpert, J.; Hirth, V.; Wieland, D.; Eleazer, G.P. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M414–M418. [Google Scholar] [CrossRef]
- Haag, M.D.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.; Breteler, M.M. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The rotterdam study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Wanamaker, B.L.; Swiger, K.J.; Blumenthal, R.S.; Martin, S.S. Cholesterol, statins, and dementia: What the cardiologist should know. Clin. Cardiol. 2015, 38, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N.; Kurinami, H.; Takeuchi, D.; Takeda, S.; Shimamura, M.; Yamashita, T.; Uchiyama, Y.; Rakugi, H.; Morishita, R. Reduction of brain β-amyloid (Aβ) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFS) and Aβ clearance. J. Biol. Chem. 2010, 285, 22091–22102. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Larson, E.B.; Sonnen, J.A.; Shofer, J.B.; Petrie, E.C.; Schantz, A.; Peskind, E.R.; Raskind, M.A.; Breitner, J.C.; Montine, T.J. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 2007, 69, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Fan, Y.C.; Wang, M.; Wang, D.; Li, X.H. Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-α in the hippocampus of an amyloid β1–42-induced rat model of Alzheimer’s disease. Clin. Interv. Aging 2013, 8, 103–110. [Google Scholar] [PubMed]
- Barone, E.; Cenini, G.; Di Domenico, F.; Martin, S.; Sultana, R.; Mancuso, C.; Murphy, M.P.; Head, E.; Butterfield, D.A. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: A novel mechanism of action. Pharmacol. Res. 2011, 63, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kurata, T.; Kawai, H.; Miyazaki, K.; Kozuki, M.; Morimoto, N.; Ohta, Y.; Ikeda, Y.; Abe, K. Statins have therapeutic potential for the treatment of Alzheimer’s disease, likely via protection of the neurovascular unit in the AD brain. J. Neurol. Sci. 2012, 322, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.K.; Nicolakakis, N.; Fernandes, P.; Ongali, B.; Brouillette, J.; Quirion, R.; Hamel, E. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol. Dis. 2009, 35, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Y.; He, P.; Li, D. Probucol inhibited Nox2 expression and attenuated podocyte injury in type 2 diabetic nephropathy of db/db mice. Biol. Pharm. Bull. 2013, 36, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Colle, D.; Santos, D.B.; Moreira, E.L.; Hartwig, J.M.; dos Santos, A.A.; Zimmermann, L.T.; Hort, M.A.; Farina, M. Probucol increases striatal glutathione peroxidase activity and protects against 3-nitropropionic acid-induced pro-oxidative damage in rats. PLoS ONE 2013, 8, e67658. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.; Miron, J.; Picard, C.; Gormley, P.; Theroux, L.; Breitner, J.; Dea, D. Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. 2), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.B.; Peres, K.C.; Ribeiro, R.P.; Colle, D.; dos Santos, A.A.; Moreira, E.L.; Souza, D.O.; Figueiredo, C.P.; Farina, M. Probucol, a lipid-lowering drug, prevents cognitive and hippocampal synaptic impairments induced by amyloid β peptide in mice. Exp. Neurol. 2012, 233, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.B.; Colle, D.; Moreira, E.L.; Peres, K.C.; Ribeiro, R.P.; Dos Santos, A.A.; de Oliveira, J.; Hort, M.A.; de Bem, A.F.; Farina, M. Probucol mitigates streptozotocin-induced cognitive and biochemical changes in mice. Neuroscience 2015, 284, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Graham, S.E.; Amy, R.M.; Dolphin, P.J. Cardioprotective effect of probucol in the atherosclerosis-prone JCR:LA-cp rat. Eur. J. Pharmacol. 1998, 350, 203–210. [Google Scholar] [CrossRef]
- Tenenbaum, A.; Fisman, E.Z. Fibrates are an essential part of modern anti-dyslipidemic arsenal: Spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc. Diabetol. 2012, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, D.; Das, N.R.; Gangwal, R.P.; Damre, M.V.; Sangamwar, A.T.; Sharma, S.S. Neuroprotective potential of peroxisome proliferator activated receptor-α agonist in cognitive impairment in Parkinson’s disease: Behavioral, biochemical, and PBPK profile. PPAR Res. 2014, 2014, 753587. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Payne, V.; Peiffer, A.M.; Hsu, F.C.; Riddle, D.R.; Zhao, W.; Chan, M.D.; Metheny-Barlow, L.; Robbins, M.E. The peroxisomal proliferator-activated receptor (PPAR) α agonist, fenofibrate, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat. Res. 2014, 181, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.L.; Carriere, I.; Barberger-Gateau, P.; Auriacombe, S.; Rouaud, O.; Fourlanos, S.; Berr, C.; Dupuy, A.M.; Ritchie, K. Lipid lowering agents, cognitive decline, and dementia: The three-city study. J. Alzheimers Dis. 2012, 30, 629–637. [Google Scholar] [PubMed]
- Zaminelli, T.; Gradowski, R.W.; Bassani, T.B.; Barbiero, J.K.; Santiago, R.M.; Maria-Ferreira, D.; Baggio, C.H.; Vital, M.A. Antidepressant and antioxidative effect of ibuprofen in the rotenone model of Parkinson’s disease. Neurotox. Res. 2014, 26, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Int’ Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Szekely, C.A.; Zandi, P.P. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: The epidemiological evidence. CNS Neurol. Disord. Drug Targets 2010, 9, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, L.; Ongini, E.; Wenk, G. Non-steroidal anti-inflammatory drugs (NSAIDS) in Alzheimer’s disease: Old and new mechanisms of action. J. Neurochem. 2004, 91, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000, 20, 5709–5714. [Google Scholar] [PubMed]
- Zara, S.; De Colli, M.; Rapino, M.; Pacella, S.; Nasuti, C.; Sozio, P.; Di Stefano, A.; Cataldi, A. Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer’s disease rat model. Gerontology 2013, 59, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Otani, M.; Takano, M.; Kadoyama, K.; Matsuyama, S. The influence of chronic ibuprofen treatment on proteins expressed in the mouse hippocampus. Eur. J. Pharmacol. 2015, 752, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Carreras, I.; Aytan, N.; Jenkins-Sahlin, E.; Dedeoglu, A.; Jenkins, B.G. The effects of aging, housing and ibuprofen treatment on brain neurochemistry in a triple transgene Alzheimer’s disease mouse model using magnetic resonance spectroscopy and imaging. Brain Res. 2014, 1590, 85–96. [Google Scholar] [CrossRef] [PubMed]
| Antioxidants | Condition | Study Type/Model | Neuronal Measure(s) | Blood-Brain Barrier Measure(s) | Others | Reference No. |
|---|---|---|---|---|---|---|
| Vitamin C&E | cerebral ischemia | mouse model | ↓ neuronal loss | ↑ claudin-5 | [54] | |
| AD | mouse model (5XFAD) | ↓ amyloid plaques | ↓ BBB dysfunction | [51] | ||
| BBB disruption | mouse model | N/A | ↑ occludin, claudin-5 | [52] | ||
| stroke | rat model | N/A | ↓ BBB dysfunction | [53] | ||
| hyperglycemia | HBMEC | N/A | ↓ BBB dysfunction | [55] | ||
| Vitamin E | Phospholipid transfer protein deficient | mouse model | N/A | ↑ occludin, claudin-5, ZO-1 | [56] | |
| healthy | rats under hyperthermic convulsion | N/A | ↓ BBB dysfunction | [57] | ||
| healthy | rats with vitamin E deficient diet | N/A | ↓ BBB dysfunction | [58] | ||
| Melatonin | hypobaric hypoxia | rats | ↑ cognitive function; ↓ neuronal loss, neuroinflammation | ↓ BBB dysfunction | [61] | |
| inflammation | rat brain microvascular endothelial cells | N/A | ↑ ZO-1 | [62] | ||
| oxidative stress | bEnd.3 cells | N/A | ↑ claudin-5; ↓ cell death | [63] | ||
| α-Lipoic acid | high-fat diet | mouse model | ↓ neuroinflammation | ↓ BBB dysfunction | [64] | |
| ischemic stroke | rat model | ↓ neurological deficit, neuroinflammation | ↓ BBB dysfunction | [65] | ||
| Aged garlic extract | high-fat diet | mouse model | ↓ neuroinflammation | ↓ BBB dysfunction | [64] | |
| Apocynin | BBB disruption | rat perfusion model | N/A | ↓ BBB dysfunction | improved vascular tone | [66] |
| BBB disruption | HBMEC | N/A | ↓ BBB dysfunction | ↑ AMPK activation | [67] | |
| BBB disruption | HBMEC | N/A | ↑ occludin, claudin-5 | ↑ AMPK activation | [68] | |
| Baicalein | intracerebral hemorrhage | rat model | ↓ neurological deficit | ↑ ZO-1 | [69] | |
| Caffein | Parkinson’s disease | mouse model | N/A | ↑ occludin, ZO-1 | [70] | |
| AD | rabbit model | ↓ neuroinflammation | ↑ occludin, ZO-1 | [71] | ||
| Curcumin | subarachnoid hemorrhage | rat model | ↓ neurological deficit, neuroinflammation | ↓ BBB dysfunction | [72] | |
| cerebral ischemia | rat model | ↓ neurological deficit | ↓ BBB dysfunction | ↓ infarct volume | [73] | |
| N/A | BMEC | N/A | ↓ platelet recruitment | [74] | ||
| Pinocembrin | cerebral ischemia | rat model | ↓ neurological deficit | ↓ BBB dysfunction | ↓ brain edema | [75] |
| cerebral ischemia | rat model | ↓ neuroinflammation | ↑ occludin, ZO-1 | [76] | ||
| Resveratrol | autoimmune encephalomyelitis | mouse model | ↓ neuroinflammation, oxidative stress | ↑ occludin, ZO-1, claudin-5; ↓ ICAM-1, VCAM-1 | [77] | |
| BBB disruption | HBMEC | ↓ oxidative stress | ↓ BBB dysfunction | [78] | ||
| AD | rat model | ↓ neuroinflammation, β-amyloid | ↑ claudin-5 | [79] | ||
| cerebral ischemia | rat model | ↓ neuronal loss | ↓ BBB dysfunction | ↓ brain edema | [80] | |
| high-fat diet | mouse model | ↓ neuronal loss | ↑ occludin, ZO-1 | [81] | ||
| Tanshinone IIA | autoimmune encephalomyelititis | mouse model | ↓ neuroinflammation | ↑ occludin, claudin-5, ZO-1 | [82] | |
| hypoxia | HBMEC | N/A | ↑ ZO-1 | [83] | ||
| cerebral ischemia | rat model | N/A | ↑ occludin, ZO-1; ↓ ICAM-1 | ↓ brain edema | [84] | |
| Statin | AD | in vitro BBB model | N/A | ↓ BBB dysfunction | [85] | |
| high-fat diet | mouse model | N/A | ↓ BBB dysfunction | [86] | ||
| Probucol | high-fat diet | mouse model | ↓ neuroinflammation | ↓ BBB dysfunction | [87,88] | |
| Fenofibrate | BBB disruption | mouse model | ↓ neurodegeneration, neuroinflammation | ↓ BBB dysfunction | [89] | |
| BBB disruption | BMEC | N/A | ↓ BBB dysfunction | [90] | ||
| Ibuprofen | high-fat diet | mouse model | N/A | ↓ BBB dysfunction | [86] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, V.; Hackett, M.; Takechi, R. Antioxidants and Dementia Risk: Consideration through a Cerebrovascular Perspective. Nutrients 2016, 8, 828. https://doi.org/10.3390/nu8120828
Lam V, Hackett M, Takechi R. Antioxidants and Dementia Risk: Consideration through a Cerebrovascular Perspective. Nutrients. 2016; 8(12):828. https://doi.org/10.3390/nu8120828
Chicago/Turabian StyleLam, Virginie, Mark Hackett, and Ryusuke Takechi. 2016. "Antioxidants and Dementia Risk: Consideration through a Cerebrovascular Perspective" Nutrients 8, no. 12: 828. https://doi.org/10.3390/nu8120828




