Crucial Role of Vitamin D in the Musculoskeletal System
Abstract
:1. Introduction
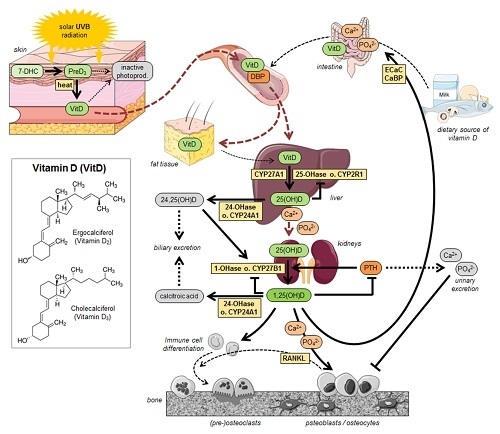
2. Vitamin D—Chemistry, Physiology and Metabolism
3. Vitamin D—Prevalence of Deficiency and Insufficiency
4. Bone Health
5. Vitamin D and Sarcopenia
6. Chronic Diseases as Risk Factor for Vitamin D Deficiency and Reduced Bone Strength (Secondary Osteoporosis)
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1,25(OH)D | 1,25-dihydroxyvitamin D |
| 1-OHase | 25-hydroxyvitamn D-1-hydroxylase |
| 24OHase | enzyme 25-hydroxyvitamin D3-24-hydroxylase |
| 25(OH)D | 25-dihydroxyvitamin D |
| 7-DHC | 7-Dehydrocholesterol |
| BMD | bone mineral density |
| DBP | vitamin D-binding protein |
| DHCR7 | 7-Dehydrocholesterol-reductase |
| DPYD | deoxypyridinoline |
| DXA | dual-energy X-ray absorptiometry |
| FGF-3 | fibroblast growth factors |
| PTH | parathyroid hormone |
| RANK | receptor activator of NFkB |
| RANKL | receptor activator of NFKB ligand |
| RXR | retinoid × receptor |
| UV | ultraviolet |
| VDR | vitamin D receptor |
| VDREs | vitamin D response elements |
References
- Funk, C. On the chemical nature of the substance which cures polyneuritis in birds induced by a diet of polished rice. J. Physiol. 1911, 43, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Deluca, H.F. Biologically active metabolite of vitamin D3 from bone liver and blood serum. J. Lipid. Res. 1966, 7, 739–744. [Google Scholar] [PubMed]
- Deluca, H.F. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, O.; Itzhaki, D.; Makarov, S.; Bennun, M.; Amital, H. A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus 2015, 24, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Heath, K.M.; Elovic, E.P. Vitamin D deficiency—Implications in the rehabilitation setting. Am. J. Phys. Med. Rehab. 2006, 85, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Shinchuk, L.; Holick, M.F. Vitamin D and rehabilitation: Improving functional outcomes. Nutr. Clin. Pract. 2007, 22, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Makitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; He, D.H.; Zhou, B.; Zhu, Y.B.; Zhao, D.; Huang, L.C.; Ding, G.Q. Analysis of vitamin D status in men highly exposed to sunlight. Biomed. Environ. Sci. 2015, 28, 913–916. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D: A d-lightful solution for health. J. Invest. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef]
- Knutsen, K.V.; Brekke, M.; Gjelstad, S.; Lagerlov, P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: A cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand. J. Prim. Health 2010, 28, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Reuss-Borst, M.A. Metabolic bone disease osteomalacia. Z. Rheumatol. 2014, 73, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Barvencik, F.; Amling, M. Vitamin D metabolism of the bone. Orthopade 2015, 44, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Hypponen, E.; Saarnio, J.; Jarvelin, M.R.; Herzig, K.H. Vitamin D and adipose tissue—More than storage. Front. Physiol. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.L.M.; MacDonald, P.N. Vitamin D: More than a "bone-a-fide" hormone. Mol. Endocrinol. 2003, 17, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.S.; Brown, A.J. Direct suppression of Pth gene expression by the vitamin D prohormones doxercalciferol and calcidiol requires the vitamin D receptor. J. Mol. Endocrinol. 2011, 46, 63–66. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ganesh, H.K.; Acharya, S.; Bandgar, T.R.; Shivane, V.; Karvat, A.; Bhatia, S.J.; Shah, S.; Menon, P.S.; Shah, N. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J. Gastroenterol. 2009, 15, 3516–3522. [Google Scholar] [CrossRef] [PubMed]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. Pth increases fgf23 gene expression and mediates the high–fgf23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal. 2010, 299, F882–F889. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.H.; Apalset, E.M.; Nordbo, Y.; Varhaug, J.E.; Mellgren, G.; Lien, E.A. 1,25-dihydroxyvitamin D and the vitamin D receptor gene polymorphism apa1 influence bone mineral density in primary hyperparathyroidism. PLoS ONE 2013, 8, e56019. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, V.; Qian, Q. Calcium regulation and bone mineral metabolism in elderly patients with chronic kidney disease. Nutrients 2013, 5, 1913–1936. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Tomar, M.; Chawla, Y.K.; Bhadada, S.K.; Khandelwal, N.; Dhiman, R.K.; Duseja, A.; Bhansali, A. Hepatic osteodystrophy is common in patients with noncholestatic liver disease. Dig. Dis. Sci. 2011, 56, 3323–3327. [Google Scholar] [CrossRef] [PubMed]
- Servier Medical Art. Powerpoint Image Bank. Available online: http://www.servier.com/Powerpoint–image-bank (accessed on 17 May 2016).
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. 2009, 12, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Umhau, J.C.; George, D.T.; Heaney, R.P.; Lewis, M.D.; Ursano, R.J.; Heilig, M.; Hibbeln, J.R.; Schwandt, M.L. Low vitamin D status and suicide: A case-control study of active duty military service members. PLoS ONE 2013, 8, e51543. [Google Scholar] [CrossRef]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014, 3, 495. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Siragy, H.M. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol. Met. 2003, 14, 274–281. [Google Scholar] [CrossRef]
- Williams, S.; Malatesta, K.; Norris, K. Vitamin D and chronic kidney disease. Ethn. Dis. 2009, 19, As8–As11. [Google Scholar]
- Putz-Bankuti, C.; Pilz, S.; Stojakovic, T.; Scharnagl, H.; Pieber, T.R.; Trauner, M.; Obermayer-Pietsch, B.; Stauber, R.E. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012, 32, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C. Renoprotective effects of vitamin D analogs. Kidney Int. 2010, 78, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Callegari, E.T.; Reavley, N.; Garland, S.M.; Gorelik, A.; Wark, J.D.; Team, S.-D.S. Vitamin D status, bone mineral density and mental health in young Australian women: The safe-D study. J. Public Health Res. 2015, 4, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Arnold, D.; Qiu, C.F.; Miller, R.S.; Williams, M.A.; Enquobahrie, D.A. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J. Womens Health 2014, 23, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports health benefits of vitamin D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Masood, T.; Kushwaha, R.S.; Singh, R.; Sailwal, S.; Pandey, H.; Varma, A.; Singh, R.K.; Cornelissen, G. Circadian rhythm of serum 25 (OH) vitamin D, calcium and phosphorus levels in the treatment and management of type-2 diabetic patients. Drug Discov. Ther. 2015, 9, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011, 59, 1–8. [Google Scholar] [PubMed]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Rieckmann, N.; Hintzpeter, B.; Mensink, G.B.M. Vitamin D status among adults in Germany—Results from the German health interview and examination survey for adults (DEGS1). BMC Public Health 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Amling, M. Calcium and vitamin D in bone metabolism. Clinical importance for fracture treatment. Unfallchirurg 2015, 118, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Sun exposure and vitamin D sufficiency. Am. J. Clin. Nutr. 2008, 88, 570S–577S. [Google Scholar] [PubMed]
- El-Sonbaty, M.R.; Abdul-Ghaffar, N.U. Vitamin D deficiency in veiled Kuwaiti women. Eur. J. Clin. Nutr. 1996, 50, 315–318. [Google Scholar] [PubMed]
- Diamond, T.H.; Levy, S.; Smith, A.; Day, P. High bone turnover in muslim women with vitamin D deficiency. Med. J. Aust. 2002, 177, 139–141. [Google Scholar] [PubMed]
- Allali, F.; el Aichaoui, S.; Saoud, B.; Maaroufi, H.; Abouqal, R.; Hajjaj-Hassouni, N. The impact of clothing style on bone mineral density among post menopausal women in Morocco: A case–control study. BMC Public Health 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D. The pathophysiology of osteoporotic hip fracture. Mcgill J. Med. 2008, 11, 51–57. [Google Scholar] [PubMed]
- Rader, C.P.; Corsten, N.; Rolf, O. Osteomalacia and vitamin D deficiency. Orthopade 2015, 44, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Staehelin, H.B.; Orav, J.E.; Stuck, A.E.; Theiler, R.; Wong, J.B.; Egli, A.; Kiel, D.P.; Henschkowski, J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. Br. Med. J. 2009, 339, 843–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernazza, P.; Stoliaroff, A.; Boggian, K.; Schlegel, M. Vitamin D Substitution. Available online: https://www.guidelines.ch/page/pdf/53 (accessed on 17 May 2016).
- DVO Leitlinie Osteoporose 2014 Kurzfassung und Langfassung. Available online: http://www.dv–osteologie.org/uploads/Leitlinie%202014/DVO–Leitlinie%20Osteoporose%202014%20Kurzfassung%20und%20Langfassung%20Version%201a%2012%2001%202016.pdf (accessed on 17 May 2016).
- Gesundes Altern: Höhere Vitamin–D Dosis Kann Sturzrisiko Erhöhen. Available online: http://www.aerzteblatt.de/nachrichten/65299/Gesundes–Altern–Hoehere–Vitamin–D–Dosis–kann–Sturzrisiko–erhoehen (accessed on 17 May 2016).
- Sharifi, N.; Amani, R.; Hajiani, E.; Cheraghian, B. Women may respond different from men to vitamin D supplementation regarding cardiometabolic biomarkers. Exp. Biol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, J.L.H.; Bijvoet, O.L.M. Rickets before the discovery of vitamin D. Bone Key Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bivins, R. Ideology and disease identity: The politics of rickets, 1929–1982. Med. Humanit. 2014, 40, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.M. Sir robert hutchison (1871–1960) of London and the causes and treatment of rickets. Arch. Dis. Child. Fetal 2005, 90, F537–F539. [Google Scholar] [CrossRef] [PubMed]
- Goldacre, M.; Hall, N.; Yeates, D.G.R. Hospitalisation for children with rickets in England: A historical perspective. Lancet 2014, 383, 597–598. [Google Scholar] [CrossRef]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia-proposal by an expert panel supported by the ministry of health, labour and welfare, Japan, the Japanese society for bone and mineral research, and the Japan endocrine society. J. Bone Miner. Metab. 2015, 33, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, M.; Daolio, P.A.; Rancati, J.M.; Ligabue, N.; Andreolli, A.; Panella, L. Rehabilitation of a patient receiving a large–resection hip prosthesis because of a phosphaturic mesenchymal tumor. Clin. Pract. 2015, 5, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Arantes, H.P.; Gimeno, S.G.; Chiang, A.Y.; Bilezikian, J.P.; Lazaretti-Castro, M. Incidence of vertebral fractures in calcium and vitamin D–supplemented postmenopausal Brazilian women with osteopenia or osteoporosis: Data from arzoxifene generations trial. Arch. Endocrinol. Metab. 2016, 60, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Siris, E.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.; Harris, S.; Jan de Beur, S.; Khosla, S.; Lane, N.; et al. The clinical diagnosis of osteoporosis: A position statement from the national bone health alliance working group. Osteoporo. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Moon, S.H.; Ha, Y.C.; Lee, D.Y.; Gong, H.S.; Park, S.Y.; Yang, K.H. Osteoporotic fracture: 2015 position statement of the Korean society for bone and mineral research. J. Bone Metab. 2015, 22, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American society for bone and mineral research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.D.; LaCroix, A.Z.; Gass, M.; Wallace, R.B.; Robbins, J.; Lewis, C.E.; Bassford, T.; Beresford, S.A.; Black, H.R.; Blanchette, P.; et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006, 354, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Rode, A.; Fourlanos, S.; Nicoll, A. Oral vitamin D replacement is effective in chronic liver disease. Gastroenterol. Clin. Biol. 2010, 34, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut. Prävalenz von Osteoporose; Robert–Koch–Institut: Berlin, Germany, 2009; pp. 97–99. [Google Scholar]
- Servier Medical Art. Bone Structure. Available online: http://www.servier.com/slidekit/?item=2 (accessed on 17 May 2016).
- Winzenberg, T.; van der Mei, I.; Mason, R.S.; Nowson, C.; Jones, G. Vitamin D and the musculoskeletal health of older adults. Aust. Fam. Physician 2012, 41, 92–99. [Google Scholar] [PubMed]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Orav, E.J.; Lips, P.; Meunier, P.J.; Lyons, R.A.; Flicker, L.; Wark, J.; Jackson, R.D.; Cauley, J.A.; et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N. Engl. J. Med. 2012, 367, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Baron, J.A.; Burckhardt, P.; Li, R.; Spiegelman, D.; Specker, B.; Orav, J.E.; Wong, J.B.; Staehelin, H.B.; et al. Calcium intake and hip fracture risk in men and women: A meta-analysis of prospective cohort studies and randomized controlled trials. Am. J. Clin. Nutr. 2007, 86, 1780–1790. [Google Scholar] [PubMed]
- Cummings, S.R.; Kiel, D.P.; Black, D.M. Vitamin D supplementation and increased risk of falling: A cautionary tale of vitamin supplements retold. JAMA Intern. Med. 2016, 176, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Montero-Odasso, M.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of osteosarcopenia in older individuals with a history of falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Baek, K.H.; Song, K.H.; Kang, M., II; Park, C.Y.; Lee, W.Y.; Oh, K.W. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: The fourth Korea national health and nutrition examination surveys (KNHANES IV) 2009. J. Clin. Endocr. Metab. 2011, 96, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Snijder, M.B.; van Schoor, N.M.; Pluijm, S.M.F.; van Dam, R.M.; Visser, M.; Lips, P. Vitamin D status in relation to one–year risk of recurrent falling in older men and women. J. Clin. Endocr. Metab. 2006, 91, 2980–2985. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W. Closing in on vitamin D action in skeletal muscle: Early activity in muscle stem cells? Endocrinology 2016, 157, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Johnson, C.A.; McCabe, G.P.; Carnell, N.S. Dietary protein requirements of younger and older adults. Am. J. Clin. Nutr. 2008, 88, 1322–1329. [Google Scholar] [PubMed]
- De Koning, E.J.; van Schoor, N.M.; Penninx, B.W.J.H.; Elders, P.J.M.; Heijboer, A.C.; Smit, J.H.; Bet, P.M.; van Tulder, M.W.; den Heijer, M.; van Marwijk, H.W.J.; et al. Vitamin D supplementation to prevent depression and poor physical function in older adults: Study protocol of the D-vitaal study, a randomized placebo-controlled clinical trial. BMC Geriatr. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Orav, E.J.; Staehelin, H.B.; Meyer, O.W.; Theiler, R.; Dick, W.; Willett, W.C.; Egli, A. Monthly high-dose vitamin D treatment for the prevention of functional decline: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 175–183. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.S.; Pye, S.R.; Mc Beth, J.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Bouillon, R.; Casanueva, F.; Finn, J.D.; et al. Low vitamin D and the risk of developing chronic widespread pain: Results from the european male ageing study. BMC Musculoskelet. Dis. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E. Hepatic osteodystrophy—Vitamin-D metabolism in patients with liver–disease. Gut 1986, 27, 1073–1090. [Google Scholar] [CrossRef] [PubMed]
- Mounach, A.; Ouzzif, Z.; Wariaghli, G.; Achemlal, L.; Benbaghdadi, I.; Aouragh, A.; Bezza, A.; el Maghraoui, A. Primary biliary cirrhosis and osteoporosis: A case–control study. J. Bone Miner. Metab. 2008, 26, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Arteh, J.; Narra, S.; Nair, S. Prevalence of vitamin D deficiency in chronic liver disease. Digest. Dis. Sci. 2010, 55, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, Y.; Hou, Y.; Han, F.; Ren, J.; Hu, Z. Vitamin D deficiency and related risk factors in patients with diabetic nephropathy. J. Int. Med. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Freude, T.; Ihle, C.; Mayer, L.; Braun, B.; Graeser, J.; Flesch, I.; Stockle, U.; Nussler, A.K.; Pscherer, S. Factors circulating in the blood of type 2 diabetes mellitus patients affect osteoblast maturation—Description of a novel in vitro model. Exp. Cell Res. 2015, 332, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Nussler, A.K.; Wildemann, B.; Freude, T.; Litzka, C.; Soldo, P.; Friess, H.; Hammad, S.; Hengstler, J.G.; Braun, K.F.; Trak-Smayra, V.; et al. Chronic CCl4 intoxication causes liver and bone damage similar to the human pathology of hepatic osteodystrophy: A mouse model to analyse the liver-bone axis. Arch. Toxicol. 2014, 88, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.T.; Braun, K.F.; Ehnert, S.; Mayer, L.; Stockle, U.; Nussler, A.K.; Pscherer, S.; Freude, T. Gene expression changes in cancellous bone of type 2 diabetics: A biomolecular basis for diabetic bone disease. Langenbeck Arch. Surg. 2014, 399, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hochrath, K.; Ehnert, S.; Ackert-Bicknell, C.L.; Lau, Y.; Schmid, A.; Krawczyk, M.; Hengstler, J.G.; Dunn, J.; Hiththetiya, K.; Rathkolb, B.; et al. Modeling hepatic osteodystrophy in Abcb4 deficient mice. Bone 2013, 55, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.X.; Armas, L.; Lappe, J.; Zhou, Y.; Gao, G.M.; Wang, H.W.; Recker, R.; Zhao, L.J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy caucasian subjects. Hum. Genet. 2010, 128, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Expanding role for vitamin D in chronic kidney disease: Importance of blood 25-OH-D levels and extra-renal 1 alpha-hydroxylase in the classical and nonclassical actions of 1 alpha,25-dihydroxyvitamin D-3. Semin. Dial. 2007, 20, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Borozan, S.; Radojevic, N.; Aligrudic, S.; Bozovic, D. Relationship between 25-hydroxyvitamin D and newly diagnosed type 2 diabetes mellitus in postmenopausal women with osteoporosis. Med. Princ. Pract. 2014, 23, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Hogler, W.; Baumann, U.; Kelly, D. Endocrine and bone metabolic complications in chronic liver disease and after liver transplantation in children. J. Pediatr. Gastr. Nutr. 2012, 54, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, N.M.; Sakhaee, K. Treatment of osteoporosis in patients with chronic liver disease and in liver transplant recipients. Curr. Treat. Options Gastroenterol. 2006, 9, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Yurci, A.; Kalkan, A.O.; Ozbakir, O.; Karaman, A.; Torun, E.; Kula, M.; Baskol, M.; Gursoy, S.; Yucesoy, M.; Bayram, F. Efficacy of different therapeutic regimens on hepatic osteodystrophy in chronic viral liver disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.R.; Nikooyeh, B.; Kalayi, A.; Zahedirad, M.; Shariatzadeh, N. A vitamin D-calcium-fortified yogurt drink decreased serum PTH but did not affect osteocalcin in subjects with type 2 diabetes. Int. J. Vitam. Nutr. Res. 2015, 85, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wariaghli, G.; Allali, F.; el Maghraoui, A.; Hajjaj-Hassouni, N. Osteoporosis in patients with primary biliary cirrhosis. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Guanabens, N.; Pares, A. Management of osteoporosis in liver disease. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rudic, J.S.; Giljaca, V.; Krstic, M.N.; Bjelakovic, G.; Gluud, C. Bisphosphonates for osteoporosis in primary biliary cirrhosis. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Luxon, B.A. Bone disorders in chronic liver diseases. Curr. Gastroenterol. Rep. 2011, 13, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.; Rubio, N.; Rubio, E.; Indreika, M.; Seitam, A. Chronic alcoholism. Frequently overlooked cause of osteoporosis in men. Am. J. Med. 1986, 80, 393–397. [Google Scholar] [CrossRef]
- Diamond, T.; Stiel, D.; Posen, S. Osteoporosis in hemochromatosis: Iron excess, gonadal deficiency, or other factors? Ann. Intern. Med. 1989, 110, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Diamond, T.H.; Stiel, D.; Lunzer, M.; McDowall, D.; Eckstein, R.P.; Posen, S. Hepatic osteodystrophy. Static and dynamic bone histomorphometry and serum bone Gla–protein in 80 patients with chronic liver disease. Gastroenterology 1989, 96, 213–221. [Google Scholar] [PubMed]
- Diamond, T.; Stiel, D.; Lunzer, M.; Wilkinson, M.; Posen, S. Ethanol reduces bone formation and may cause osteoporosis. Am. J. Med. 1989, 86, 282–288. [Google Scholar] [CrossRef]
- Diamond, T.; Stiel, D.; Lunzer, M.; Wilkinson, M.; Roche, J.; Posen, S. Osteoporosis and skeletal fractures in chronic liver disease. Gut 1990, 31, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Guanabens, N.; Pares, A.; Marinoso, L.; Brancos, M.A.; Piera, C.; Serrano, S.; Rivera, F.; Rodes, J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am. J. Gastroenterol. 1990, 85, 1356–1362. [Google Scholar] [PubMed]
- Gonzalez-Calvin, J.L.; Garcia-Sanchez, A.; Bellot, V.; Munoz-Torres, M.; Raya-Alvarez, E.; Salvatierra-Rios, D. Mineral metabolism, osteoblastic function and bone mass in chronic alcoholism. Alcohol Alcohol. 1993, 28, 571–579. [Google Scholar] [PubMed]
- Kayath, M.J.; Dib, S.A.; Vieira, J.G.H. Prevalence and magnitude of osteopenia associated with insulin- dependent diabetes-mellitus. J. Diabetes Complicat. 1994, 8, 97–104. [Google Scholar] [CrossRef]
- Lindor, K.D.; Janes, C.H.; Crippin, J.S.; Jorgensen, R.A.; Dickson, E.R. Bone disease in primary biliary cirrhosis: Does ursodeoxycholic acid make a difference? Hepatology 1995, 21, 389–392. [Google Scholar] [PubMed]
- Monegal, A.; Navasa, M.; Guanabens, N.; Peris, P.; Pons, F.; de Osaba, M.J.M.; Rimola, A.; Rodes, J.; Munoz-Gomez, J. Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif. Tissue Int. 1997, 60, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, L.; Fargion, S.; Fracanzani, A.L.; Binelli, L.; Battafarano, N.; Varenna, M.; Piperno, A.; Fiorelli, G. Bone and joint involvement in genetic hemochromatosis: Role of cirrhosis and iron overload. J. Rheumatol. 1997, 24, 1809–1813. [Google Scholar] [PubMed]
- Kayath, M.J.; Tavares, E.F.; Dib, S.A.; Vieira, J.G.H. Prospective bone mineral density evaluation in patients with insulin-dependent diabetes mellitus. J. Diabetes Complicat. 1998, 12, 133–139. [Google Scholar] [CrossRef]
- Gunczler, P.; Lanes, R.; Paz-Martinez, V.; Martinis, R.; Esaa, S.; Colmenares, V.; Weisinger, J.R. Decreased lumbar spine bone mass and low bone turnover in children and adolescents with insulin dependent diabetes mellitus followed longitudinally. J. Pediatr. Endocr. Met. 1998, 11, 413–419. [Google Scholar] [CrossRef]
- Angulo, P.; Therneau, T.M.; Jorgensen, A.; DeSotel, C.K.; Egan, K.S.; Dickson, E.R.; Hay, J.E.; Lindor, K.D. Bone disease in patients with primary sclerosing cholangitis: Prevalence, severity and prediction of progression. J. Hepatol. 1998, 29, 729–735. [Google Scholar] [CrossRef]
- Gallego-Rojo, F.J.; Gonzalez-Calvin, J.L.; Munoz-Torres, M.; Mundi, J.L.; Fernandez-Perez, R.; Rodrigo-Moreno, D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology 1998, 28, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Pollak, R.D.; Karmeli, F.; Eliakim, R.; Ackerman, Z.; Tabb, K.; Rachmilewitz, D. Femoral neck osteopenia in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1998, 93, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Kemink, S.A.; Hermus, A.R.; Swinkels, L.M.; Lutterman, J.A.; Smals, A.G. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J. Endocrinol. Investig. 2000, 23, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bollani, S.; Bettica, P.; Bevilacqua, M.; Molteni, P.; Porro, G.B. Altered bone metabolism in inflammatory bowel disease: There is a difference between crohn’s disease and ulcerative colitis. J. Intern. Med. 2000, 247, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Farias, M.L.; Coelho, H.S.; Mendonca, L.M.; Stabnov, L.M.; do Carmo d Oliveira, M.; Lamy, R.A.; Oliveira, D.S. Calcium-parathyroid hormone-vitamin D axis and metabolic bone disease in chronic viral liver disease. J. Gastroenterol. Hepatol. 2001, 16, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Menon, K.V.; Angulo, P.; Weston, S.; Dickson, E.R.; Lindor, K.D. Bone disease in primary biliary cirrhosis: Independent indicators and rate of progression. J. Hepatol. 2001, 35, 316–323. [Google Scholar] [CrossRef]
- Kim, S.; Koga, T.; Isobe, M.; Kern, B.E.; Yokochi, T.; Chin, Y.E.; Karsenty, G.; Taniguchi, T.; Takayanagi, H. Stat1 functions as a cytoplasmic attenuator of runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003, 17, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Sokhi, R.P.; Anantharaju, A.; Kondaveeti, R.; Creech, S.D.; Islam, K.K.; van Thiel, D.H. Bone mineral density among cirrhotic patients awaiting liver transplantation. Liver Transpl. 2004, 10, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Bone mineral density in patients with inflammatory bowel disease: A population-based prospective two-year follow-up study. Scand. J. Gastroenterol. 2004, 39, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.; Mega, A.; Camozzi, V.; Baldo, V.; Plebani, M.; Burra, P.; Luisetto, G. Is osteoporosis a peculiar association with primary biliary cirrhosis? World J. Gastroenterol. 2005, 11, 5347–5350. [Google Scholar] [CrossRef] [PubMed]
- Auletta, M.; Nuzzo, V.; Esposito, A.; Antonello, S.; Lupoli, G.; Federico, F.; de Puente, A. Osteoporosis in men: A study in patients affected by chronic non-advanced liver disease. Clin. Cases Miner. Bone Metab. 2005, 2, 25–28. [Google Scholar]
- Guggenbuhl, P.; Deugnier, Y.; Boisdet, J.F.; Rolland, Y.; Perdriger, A.; Pawlotsky, Y.; Chales, G. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int. 2005, 16, 1809–1814. [Google Scholar] [PubMed]
- Zali, M.; Bahari, A.; Firouzi, F.; Daryani, N.E.; Aghazadeh, R.; Emam, M.M.; Rezaie, A.; Shalmani, H.M.; Naderi, N.; Maleki, B.; et al. Bone mineral density in Iranian patients with inflammatory bowel disease. Int. J. Colorectal Dis. 2006, 21, 758–766. [Google Scholar] [PubMed]
- Hofmann, W.P.; Kronenberger, B.; Bojunga, J.; Stamm, B.; Herrmann, E.; Bucker, A.; Mihm, U.; von Wagner, M.; Zeuzem, S.; Sarrazin, C. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon alpha and ribavirin therapy. J. Viral Hepat. 2008, 15, 790–796. [Google Scholar] [PubMed]
- Lumachi, F.; Camozzi, V.; Tombolan, V.; Luisetto, G. Bone mineral density, osteocalcin, and bone-specific alkaline phosphatase in patients with insulin-dependent diabetes mellitus. Ann. N. Y. Acad. Sci. 2009, 1173, E64–E67. [Google Scholar] [PubMed]
- Malik, P.; Gasser, R.W.; Kemmler, G.; Moncayo, R.; Finkenstedt, G.; Kurz, M.; Fleischhacker, W.W. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: A cross-sectional study. Alcohol. Clin. Exp. Res. 2009, 33, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Goral, V.; Simsek, M.; Mete, N. Hepatic osteodystrophy and liver cirrhosis. World J. Gastroenterol. 2010, 16, 1639–1643. [Google Scholar] [PubMed]
- Loria, I.; Albanese, C.; Giusto, M.; Galtieri, P.A.; Giannelli, V.; Lucidi, C.; di Menna, S.; Pirazzi, C.; Corradini, S.G.; Mennini, G.; et al. Bone disorders in patients with chronic liver disease awaiting liver transplantation. Transplant. Proc. 2010, 42, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Wariaghli, G.; Mounach, A.; Achemlal, L.; Benbaghdadi, I.; Aouragh, A.; Bezza, A.; el Maghraoui, A. Osteoporosis in chronic liver disease: A case-control study. Rheumatol. Int. 2010, 30, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Wibaux, C.; Legroux-Gerot, I.; Dharancy, S.; Boleslawski, E.; Declerck, N.; Canva, V.; Mathurin, P.; Pruvot, F.R.; Cortet, B. Assessing bone status in patients awaiting liver transplantation. Jt. Bone Spine 2011, 78, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Grandison, G.A.; Fong, D.G.; Keach, J.C.; Lindor, K.D.; Bjornsson, E.; Koch, A. Bone disease in patients with primary sclerosing cholangitis. Gastroenterology 2011, 140, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, A.A.; Pascasio Acevedo, J.M.; Domingo, I.G.; Jimenez, R.G.; Martin, J.M.S.; Rios, M.T.F.; Mota, M.S.; Gallego, A.G.; Bravo, M.A.G. Prevalence and characteristics of bone disease in cirrhotic patients under evaluation for liver transplantation. Transplant. Proc. 2012, 44, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- Pardee, P.E.; Dunn, W.; Schwimmer, J.B. Non-alcoholic fatty liver disease is associated with low bone mineral density in obese children. Aliment. Pharmacol. Ther. 2012, 35, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, G.L.; Grobholz, S.; Bruckner, T.; Scheidt-Nave, C.; Nawroth, P.; Schneider, J.G. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr. Disord. 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Mathen, P.G.; Thabah, M.M.; Zachariah, B.; Das, A.K. Decreased bone mineral density at the femoral neck and lumbar spine in south Indian patients with type 2 diabetes. J. Clin. Diagn. Res. 2015, 9, OC8–OC12. [Google Scholar] [CrossRef] [PubMed]
- Chinnaratha, M.A.; Chaudhary, S.; Doogue, M.; McCormick, R.J.; Woodman, R.J.; Wigg, A.J. Prevalence of hepatic osteodystrophy and vitamin D deficiency in cirrhosis. Intern. Med. J. 2015, 45, 1230–1235. [Google Scholar] [CrossRef] [PubMed]


| Reference | n | Etiology | Prevalence of | |
|---|---|---|---|---|
| Osteopenia | Osteoporosis | |||
| Spencer et al., 1986 [94] | 96 | alcoholic liver disease | n.s. | 47% |
| Diamond et al., 1989 [95] | 22 | hemochromatosis | n.s. | 45% |
| Diamond et al., 1989 [96] | 80 | mixed etiology | n.s. | 21% |
| Diamond et al., 1989 [97] | 28 | alcoholic liver disease | 28% | 38% |
| Diamond et al., 1990 [98] | 115 | mixed etiology | n.s. | 28%–75% |
| Guañabens et al., 1990 [99] | 20 | primary biliary cirrhosis | 35% | |
| Gonzalez-Calvin et al., 1993 [100] | 39 | alcoholic liver disease | 23% | n.s. |
| Kayath et al., 1994 [101] | 90 | insulin-dependent diabetes mellitus | 34% | |
| Lindor et al., 1995 [102] | 88 | primary biliary cirrhosis | n.s. | 35% |
| Monegal et al., 1997 [103] | 58 | mixed etiology (cirrhosis) | n.s. | 43% |
| Sinigaglia et al., 1997 [104] | 32 | hemochromatosis | n.s. | 28% |
| Kayath et al., 1998 [105] | 23 | insulin-dependent diabetes mellitus | 48% | n.s. |
| Gunczler et al., 1998 [106] | 26 | insulin-dependent diabetes mellitus | 92.6% | |
| Angulo et al., 1998 [107] | 81 | primary sclerosing cholangitis | n.s. | 17% |
| Gallegy–Rojo et al., 1998 [108] | 32 | viral cirrhosis | n.s. | 53% |
| Pollak et al., 1998 [109] | 63 | inflammatory bowel disease | 42% | 41% |
| Kemink et al., 2000 [110] | 35 | insulin–dependent diabetes mellitus | 62% | - |
| Ardizzone et al., 2000 [111] | 51 | Crohn’s disease | 55% | 37% |
| 40 | ulcerative colitis | 76% | 18% | |
| Duarte et al., 2001 [112] | 100 | viral cirrhosis | n.s. | 25% |
| Menon et al., 2001 [113] | 176 | primary biliary cirrhosis | n.s. | 20% |
| Kim et al., 2003 [114] | 19 | alcoholic liver disease | 50% | 22% |
| Sokhi et al., 2004 [115] | 104 | mixed etiology (cirrhosis) | 34.6% | 11.5% |
| Jahnsen et al., 2004 [116] | 60 | Crohn’s disease | 22% | |
| 60 | ulcerative colitis | 27% | ||
| Floreani et al., 2005 [117] | 35 | primary biliary cirrhosis | n.s. | 14.2% |
| 49 | viral cirrhosis | n.s. | 14.3% | |
| Auletta et al., 2005 [118] | 30 | chronic hepatitis | 44% | 20% |
| Guggenbuhl et al., 2005 [119] | 38 | hemochromatosis | 44.7% | 34.2% |
| Zali et al., 2006 [120] | 165 | inflammatory bowel disease | 26.7% | 5.4% |
| Hofmann et al., 2008 [121] | 30 | chronic hepatitis C | 43% | 13% |
| Mounach et al., 2008 [76] | 33 | primary biliary cirrhosis | n.s. | 51.5% |
| Lumachi et al., 2009 [122] | 18 | insulin–dependent diabetes mellitus | 61.1% | n.s. |
| Malik et al., 2009 [123] | 57 | alcoholic liver disease | 17.5% | |
| George et al., 2009 [17] | 72 | viral and alcoholic (cirrhosis) | 68% | |
| Goral et al., 2010 [124] | 55 | mixed etiology (cirrhosis) | n.s. | 37% |
| Loria et al., 2010 [125] | 35 | mixed etiology (cirrhosis) | 26% | 14% |
| Wariaghli et al., 2010 [126] | 64 | mixed etiology (cirrhosis) | n.s. | 45.3% |
| Wibaux et al., 2011 [127] | 99 | mixed etiology (cirrhosis) | 35% | 38% |
| Angulo et al., 2011 [128] | 237 | primary sclerosing cholangitis | n.s. | 15%–75% |
| Choudhary et al., 2011 [21] | 115 | viral and alcoholic (cirrhosis) | 93.7%–97% | |
| Alcalde Vargas et al., 2012 [129] | 486 | mixed etiology (cirrhosis) | 22%–43% | 4%–23% |
| Pardee et al., 2012 [130] | 38 | non–alcoholic fatty liver disease | 45% | |
| Leidig et al., 2014 [131] | 139 | diabetes mellitus type 1 | n.s. | 27% |
| 243 | diabetes mellitus type 2 | n.s. | 14% | |
| Mathen et al., 2015 [132] | 150 | diabetes mellitus type 2 | 32% | 35% |
| Chinnaratha et al., 2015 [133] | 406 | mixed etiology (cirrhosis) | 46% | 21% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; De Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. https://doi.org/10.3390/nu8060319
Wintermeyer E, Ihle C, Ehnert S, Stöckle U, Ochs G, De Zwart P, Flesch I, Bahrs C, Nussler AK. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients. 2016; 8(6):319. https://doi.org/10.3390/nu8060319
Chicago/Turabian StyleWintermeyer, Elke, Christoph Ihle, Sabrina Ehnert, Ulrich Stöckle, Gunnar Ochs, Peter De Zwart, Ingo Flesch, Christian Bahrs, and Andreas K. Nussler. 2016. "Crucial Role of Vitamin D in the Musculoskeletal System" Nutrients 8, no. 6: 319. https://doi.org/10.3390/nu8060319