Introduction of Complementary Foods in a Cohort of Infants in Northeast Italy: Do Parents Comply with WHO Recommendations?
Abstract
:1. Introduction
2. Materials and Methods
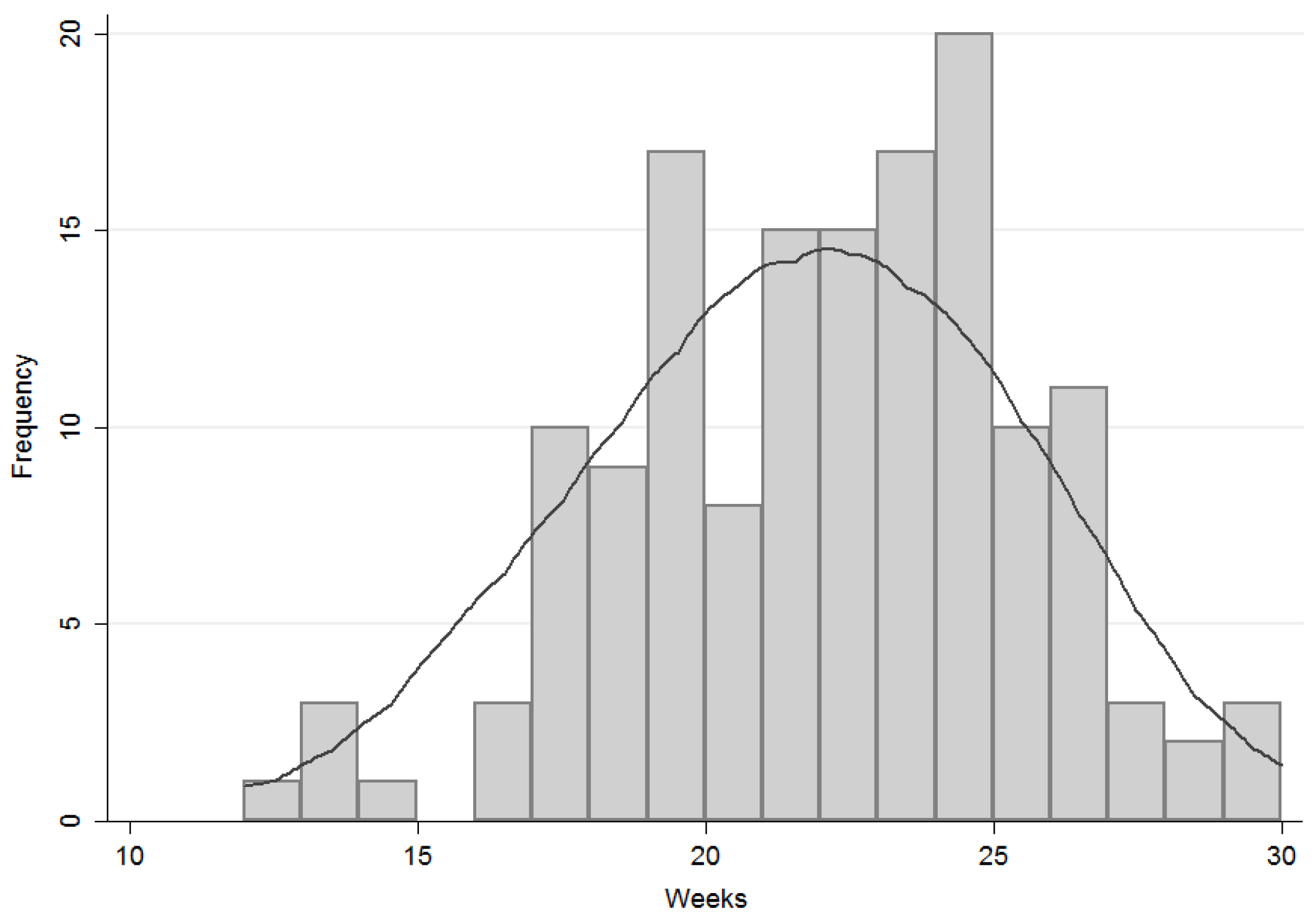
- introduction at or after 6 months;
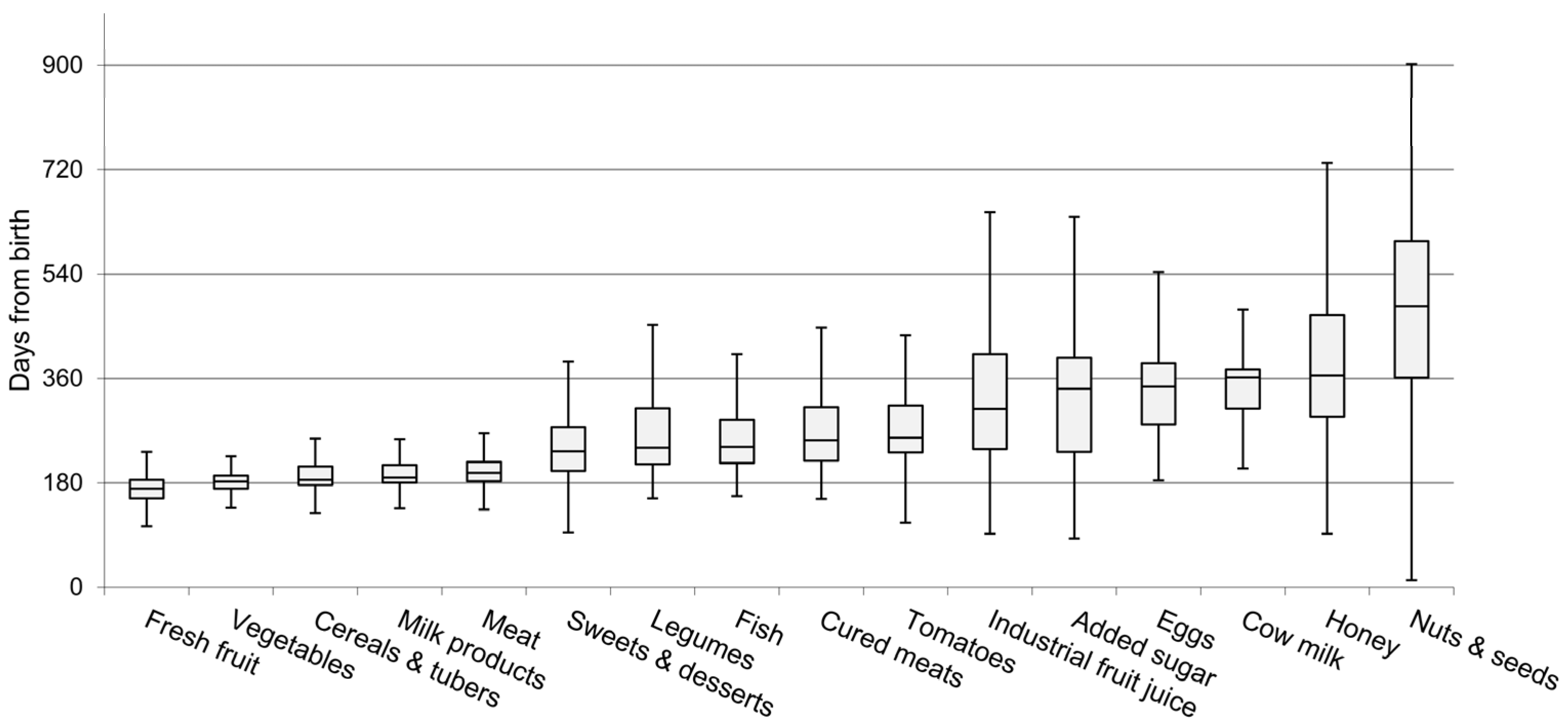
- “minimum dietary diversity”;
- use of homemade vs. commercial baby food;
- introduction of cow’s milk at 12 months or more;
- introduction of honey at 12 months or more.
3. Results
3.1. Population Characteristics
3.2. Timing of Complementary Feeding
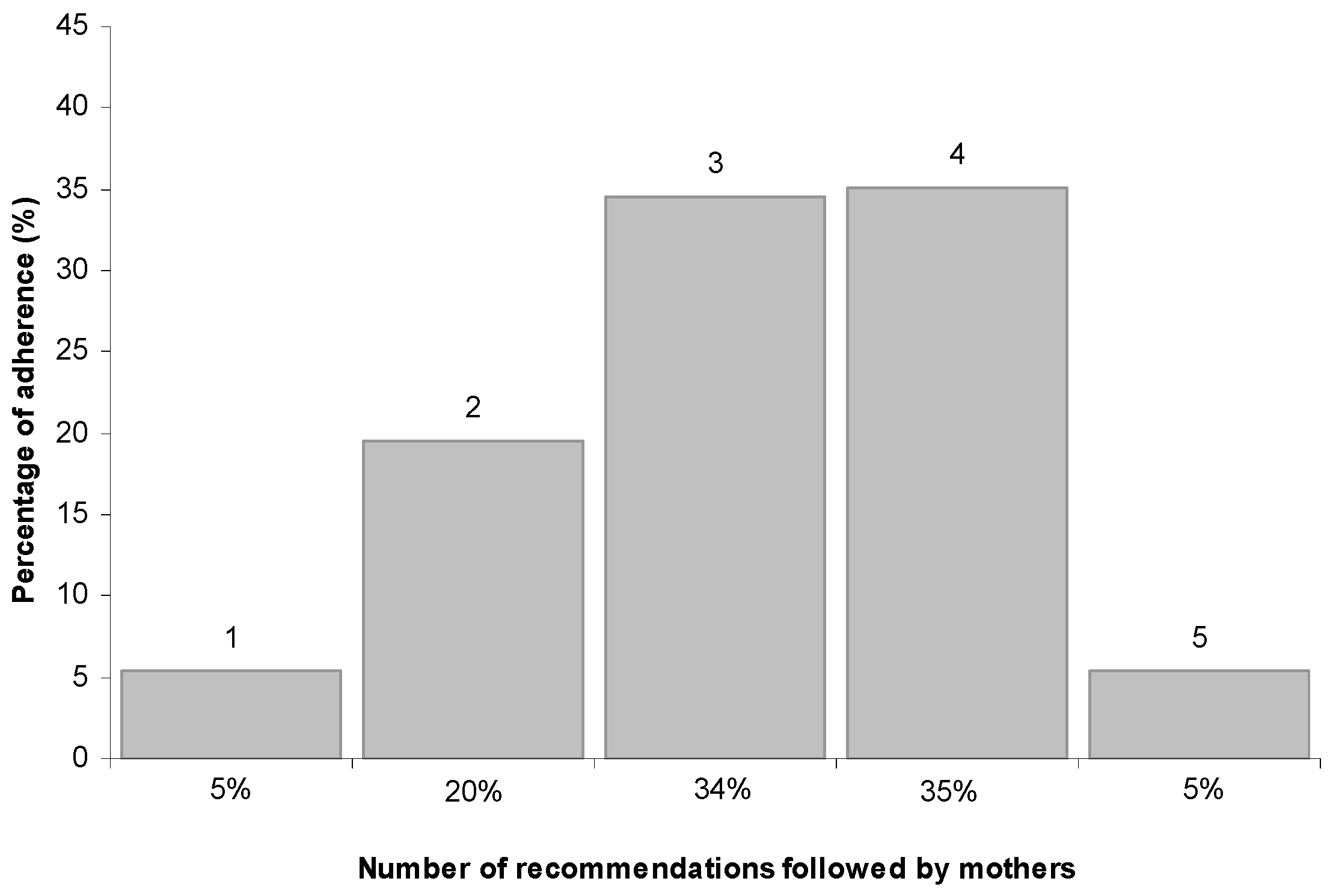
3.3. Compliance with WHO Recommendations
3.4. Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Complementay Feeding: Report of the Global Consultation, and Summary of Guiding Principles for Complementary Feeding of the Breastfed Child; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Monteiro, P.O.A.; Victora, C.G. Rapid growth in infancy and childhood and obesity in later life—A systematic review. Obes. Rev. 2005, 6, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Gray, C.; Li, M.; Segovia, S.A.; Vickers, M.H. Early Life Nutrition and Energy Balance Disorders in Offspring in Later Life. Nutrients 2015, 7, 8090–8111. [Google Scholar] [CrossRef] [PubMed]
- Ersino, G.; Henry, C.J.; Zello, G.A. Suboptimal Feeding Practices and High Levels of Undernutrition Among Infants and Young Children in the Rural Communities of Halaba and Zeway, Ethiopia. Food Nutr. Bull. 2016, 37, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Saaka, M.; Wemakor, A.; Abizari, A.R.; Aryee, P. How well do WHO complementary feeding indicators relate to nutritional status of children aged 6–23 months in rural Northern Ghana? BMC Public Health 2015, 15, 1157. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; The United Nations Children’s Fund. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Arenz, S.; Ruckerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Rifas-Shiman, S.L.; Taveras, E.M.; Oken, E.; Gillman, M.W. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011, 127, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.; Taylor, M.A.; Langley-Evans, S.C. Timing of the introduction of complementary feeding and risk of childhood obesity: A systematic review. Int. J. Obes. (Lond.) 2013, 37, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- De Beer, M.; Vrijkotte, T.G.; Fall, C.H.; van Eijsden, M.; Osmond, C.; Gemke, R.J. Associations of infant feeding and timing of linear growth and relative weight gain during early life with childhood body composition. Int. J. Obes. (Lond.) 2015, 39, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Della Salute, M. Linee di Indirizzo Nazionali Sulla Protezione, la Promozione ed il Sostegno Dell’allattamento al Seno; Gazzetta Ufficiale: Roma, Italy, 2008. [Google Scholar]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Williams, C.; Pallas-Alonso, C.R.; Hernandez-Aguilar, M.T.; Lasarte-Velillas, J.J.; Landa-Rivera, L.; Rouw, E.; Pina, M.; Volta, A.; Oudesluys-Murphy, A.M. ESPGHAN’s 2008 recommendation for early introduction of complementary foods: How good is the evidence? Matern. Child Nutr. 2011, 7, 335–343. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Exclusive Breastfeeding for Six Months Best for Babies Everywhere; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Michaelsen, K.F.; Weaver, L.; Branca, F.; Robertson, A. Feeding and Nutrition of Infants and Young Children, 87th ed.; WHO Regional Publications, European Series; WHO Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- World Health Organization. Complementary Feeding: Family Foods for Breasfed Infants; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Scott, J.A.; Binns, C.W.; Graham, K.I.; Oddy, W.H. Predictors of the early introduction of solid foods in infants: Results of a cohort study. BMC Pediatr. 2009, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tromp, I.I.M.; Briede, S.; Kiefte-de Jong, J.C.; Renders, C.M.; Jaddoe, V.W.; Franco, O.H.; Hofman, A.; Raat, H.; Moll, H.A. Factors associated with the timing of introduction of complementary feeding: The Generation R Study. Eur. J. Clin. Nutr. 2013, 67, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Riva, E.; Banderali, G.; Scaglioni, S.; Veehof, S.H.; Sala, M.; Radaelli, G.; Agostoni, C. Feeding practices of infants through the first year of life in Italy. Acta Paediatr. 2004, 93, 492–497. [Google Scholar] [PubMed]
- Pani, P.; Carletti, C.; Knowles, A.; Parpinel, M.; Concina, F.; Montico, M.; Cattaneo, A. Patterns of nutrients’ intake at six months in the northeast of Italy: A cohort study. BMC Pediatr. 2014, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Carletti, C.; Pani, P.; Knowles, A.; Monasta, L.; Montico, M.; Cattaneo, A. Breastfeeding to 24 months of age in the northeast of Italy: A cohort study. Breastfeed. Med. 2011, 6, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Schiess, S.; Grote, V.; Scaglioni, S.; Luque, V.; Martin, F.; Stolarczyk, A.; Vecchi, F.; Koletzko, B.; European Childhood Obesity Project. Introduction of complementary feeding in 5 European countries. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Biro, G.; Hulshof, K.F.; Ovesen, L.; Amorim Cruz, J.A.; EFCOSUM Group. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002, 56, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Buzzard, M. 24-Hour dietary recall and food record methods. In Nutritional Epidemiology, 2nd ed.; Willett, W., Ed.; Oxford University Press: Oxford, UK, 1998; pp. 50–73. [Google Scholar]
- World Health Organization; The United Nations Children’s Fund. Indicators for assessing infant and young child feeding practices. In Proceedings of the Conclusions of a Consensus Meeting, Washington, DC, USA, 6–8 November 2007; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- World Health Organization. Guideline: Vitamin A Supplementation in Infants and Children 6–59 Months of Age; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Clayton, H.B.; Li, R.; Perrine, C.G.; Scanlon, K.S. Prevalence and reasons for introducing infants early to solid foods: Variations by milk feeding type. Pediatrics 2013, 131, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Michaelsen, K.F.; Rasmussen, K.M.; Sørensen, T.I. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am. J. Clin. Nutr. 2004, 80, 1579–1588. [Google Scholar] [PubMed]
- Dratva, J.; Merten, S.; Ackermann-Liebrich, U. The timing of complementary feeding of infants in Switzerland: Compliance with the Swiss and the WHO guidelines. Acta Paediatr. 2006, 95, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.; Sommerville, J.; Ong, K.; Handerson, H.; Allen, R. Diet and Nutrition Survey of Infants and Young Children, 2011; Department of Health and Food Standards Agency: London, UK, 2013.
- WHO Multicenter Growth Reference Study Group. Complementary feeding in the WHO Multicenter Growth Reference Study. Acta Paediatr. 2006, 450, 27–37. [Google Scholar]
- Longo, G.; Berti, I.; Burks, A.W.; Krauss, B.; Barbi, E. IgE-mediated food allergy in children. Lancet 2013, 382, 1656–1664. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A.; INRAN-SCAI 2005-06 Study Group. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C.; INRAN-SCAI 2005-6 Study Group. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06—Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Visalli, M.; Jacob, S.; Chabanet, C.; Schlich, P.; Nicklaus, S. Maternal feeding practices during the first year and their impact on infants’ acceptance of complementary food. Food Qual. Prefer. 2013, 29, 89–98. [Google Scholar] [CrossRef]
- Schwartz, C.; Scholtens, P.A.; Lalanne, A.; Weenen, H.; Nicklaus, S. Development of healthy eating habits early in life. Review of recent evidence and selected guidelines. Appetite 2011, 57, 796–807. [Google Scholar] [PubMed]
- Nicklaus, S.; Remy, E. Early origins of overeating: Tracking between early food habits and later eating patterns. Curr. Obes. Rep. 2013, 2, 179–184. [Google Scholar] [CrossRef]
- Cashdan, E. A sensitive period for learning about food. Hum. Nat. 1994, 5, 279–291. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.M.; Murray, D.M.; Hourihane, J.O.; Kenny, L.C.; Irvine, A.D.; Kiely, M. Adherence with early infant feeding and complementary feeding guidelines in the Cork BASELINE Birth Cohort Study. Public Health Nutr. 2015, 18, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
| Cohort | Sub-Cohort | ||
|---|---|---|---|
| Mothers | n (%) | n (%) | p * |
| Age (years) | (n = 399) | (n = 148) | 0.713 |
| ≤29 | 90 (23) | 29 (20) | |
| 30–34 | 160 (40) | 59 (40) | |
| ≥35 | 149 (37) | 60 (40) | |
| Born in Italy | (n = 400) | (n = 148) | 0.451 |
| Yes | 351 (88) | 134 (91) | |
| No | 49 (12) | 14 (9) | |
| Education | (n = 348) | (n = 147) | 0.158 |
| ≤Secondary school | 59 (17) | 16 (11) | |
| Completed high school or equivalent | 154 (44) | 64 (43) | |
| Bachelor degree or higher | 135 (39) | 67 (46) | |
| Employment before birth | (n = 323) | (n = 136) | 1.000 |
| Yes | 306 (95) | 129 (95) | |
| No | 17 (5) | 7 (5) | |
| Employment at 6 months after birth | (n = 218) | (n = 147) | 0.790 |
| Yes | 176 (81) | 117 (80) | |
| No | 42 (19) | 30 (20) | |
| Allergy of mother or of other family member | (n = 263) | (n = 148) | 0.812 |
| Yes | 64 (24) | 38 (26) | |
| No | 199 (76) | 110 (74) | |
| Children | n (%) | n (%) | p |
| Gestational age (weeks) | (n = 339) | (n = 147) | 0.730 |
| 36–37 | 29 (9) | 14 (9) | |
| 38–42 | 310 (91) | 133 (91) | |
| Infant gender | (n = 345) | (n = 148) | 0.142 |
| male | 173 (50) | 85 (57) | |
| female | 172 (50) | 63 (43) | |
| Birth weight (g) | (n = 344) | (n = 148) | 0.552 |
| <2500 | 3 (1) | 2 (1) | |
| 2500–4199 | 324 (94) | 136 (92) | |
| ≥4200 | 17 (5) | 10 (7) | |
| Birth length (cm) | (n = 342) | (n = 148) | 0.563 |
| <46 | 5 (1) | 2 (1) | |
| 46–52.9 | 283 (83) | 117 (79) | |
| ≥53 | 54 (16) | 29 (20) |
| Items Included in the Score | (n) |
|---|---|
| Introduction of solid foods ≥6 months | 14% (21) |
| Reduced use of commercial baby foods | 62% (92) |
| Introduction of cow’s milk ≥12 months | 63% (94) |
| Introduction of honey ≥12 months | 80% (118) |
| Minimum dietary diversity | 96% (142) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carletti, C.; Pani, P.; Monasta, L.; Knowles, A.; Cattaneo, A. Introduction of Complementary Foods in a Cohort of Infants in Northeast Italy: Do Parents Comply with WHO Recommendations? Nutrients 2017, 9, 34. https://doi.org/10.3390/nu9010034
Carletti C, Pani P, Monasta L, Knowles A, Cattaneo A. Introduction of Complementary Foods in a Cohort of Infants in Northeast Italy: Do Parents Comply with WHO Recommendations? Nutrients. 2017; 9(1):34. https://doi.org/10.3390/nu9010034
Chicago/Turabian StyleCarletti, Claudia, Paola Pani, Lorenzo Monasta, Alessandra Knowles, and Adriano Cattaneo. 2017. "Introduction of Complementary Foods in a Cohort of Infants in Northeast Italy: Do Parents Comply with WHO Recommendations?" Nutrients 9, no. 1: 34. https://doi.org/10.3390/nu9010034






