Content Validation and Semantic Evaluation of a Check-List Elaborated for the Prevention of Gluten Cross-Contamination in Food Services
Abstract
:1. Introduction
2. Methods
2.1. Development of the Instrument
- -
- Identification/information of the establishment
- -
- Building and facilities
- -
- Equipment, furniture, and kitchenware
- -
- Food service employees
- -
- Food production and transport
- -
- Distribution
- -
- Documentation
- -
- Responsibility and authority
- -
- Coordinator of the food safety team
- -
- Internal communication
- -
- Flow charts
- -
- Traceability
- -
- Treatment of potentially unsafe products
2.2. Pilot Test (Subjective Evaluation)
2.3. Content Validation
2.4. Semantic Evaluation
2.5. Data Analysis
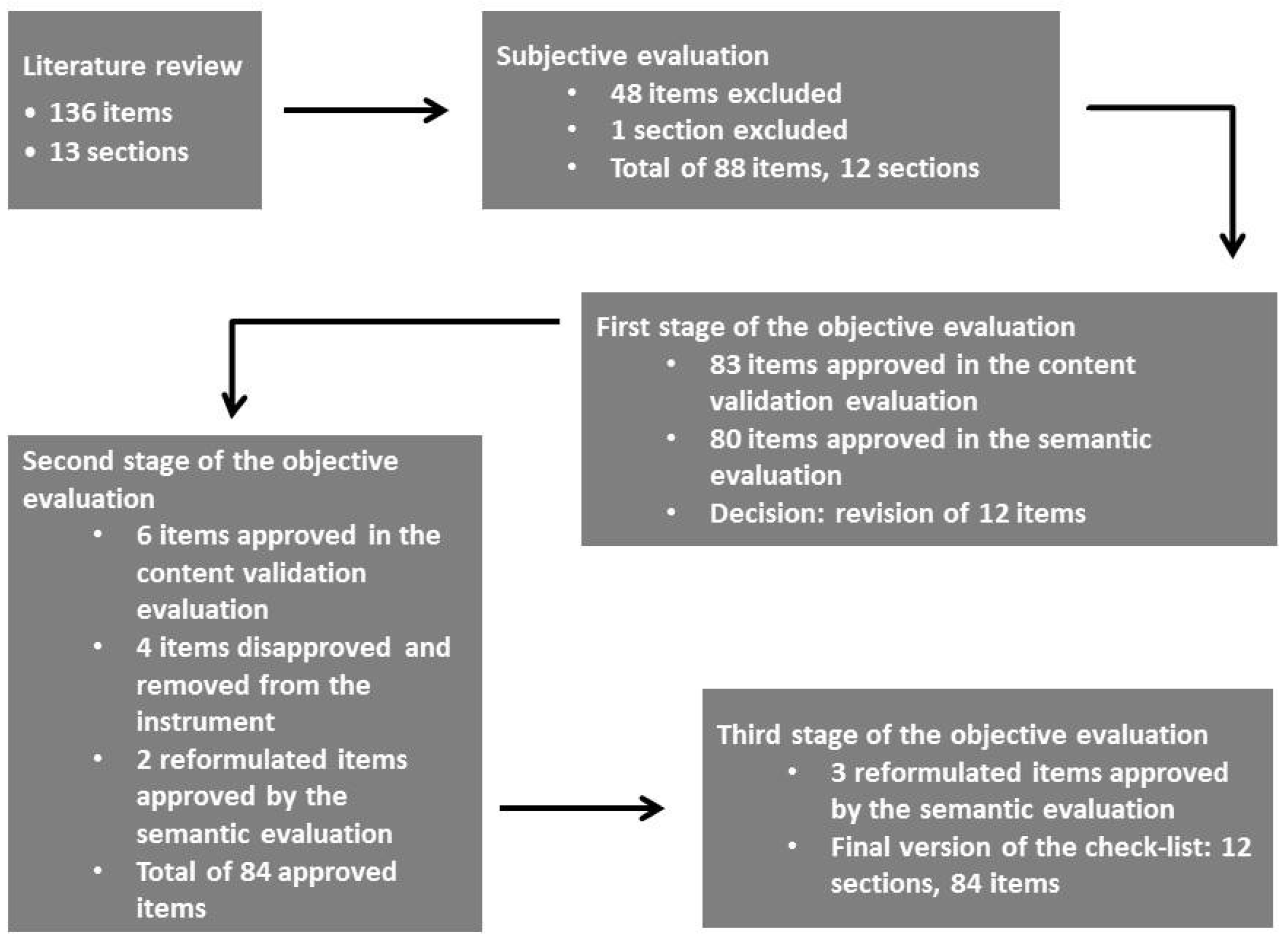
3. Results
3.1. First Stage: Content Validation and Semantic Evaluation
3.2. Second Stage: Content Validation and Semantic Evaluation
3.3. Third Stage: Semantic Evaluation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Legend: | |||
| Y—Yes | N—No | NA—Not Applicable | OBS—Observation |
| Number: | Year: | |||||||
| Company identification: | ||||||||
| Company name: | ||||||||
| Trading name: | ||||||||
| Health license: | State/municipal registration: | |||||||
| National record of legalized person/individual registration: | Phone: | Fax: | ||||||
| E-mail: | ||||||||
| Address: | ||||||||
| Neighborhood: | City: | State: | Zip code: | |||||
| Activity branch: | Monthly output: | |||||||
| Number of employees: | Number of shifts: | |||||||
| Products’ categories: | ||||||||
| Category description: | ||||||||
| Technical manager: | Academic background of the technical manager: | |||||||
| Is there an employee responsible for the good manufacturing practices in the establishment? ( ) Yes ( ) No | Academic background of the employee responsible for the good manufacturing practices: ( ) training course ( ) technical course. Which? ( ) college degree. On what? | |||||||
| Legal representative/owner of the establishment: | ||||||||
| Items | Y | N | NA | OBS | ||||
| 1. | Building and Facilities | |||||||
| 1.1. | floor | |||||||
| 1.1.1. | Floor material that allows easy and proper sanitation (smooth, drained with slope, waterproof). | |||||||
| 1.1.2. | Floor in proper conservation (free of defects, cracks, holes, and others). | |||||||
| 1.2. | Ceiling | |||||||
| 1.2.1. | Ceiling easy to clean waterproof with smooth finishing. | |||||||
| 1.3. | Walls | |||||||
| 1.3.1. | Smooth finishing walls, impermeable and easy to clean at suitable height for all operations. | |||||||
| 1.3.2. | Wall in proper conservation (free from cracks and peeling). | |||||||
| 1.4. | Doors | |||||||
| 1.4.1. | Smooth surface doors, adjusted to the jambs and without coating faults in order to reduce the risk of contamination coming from the external area. | |||||||
| 1.5. | Windows and other openings | |||||||
| 1.5.1. | Smooth surface windows, adjusted to the jambs and without coating faults in order to reduce the risk of contamination coming from the external area. | |||||||
| 1.6. | Stairs, service elevators, goods lift, and auxiliary structures | |||||||
| 1.6.1. | In case of ramps and workbenches used to support both gluten-free and gluten-containing food, a hygienic procedure is performed between the use of this surface for gluten-containing and gluten-free food. | |||||||
| 1.6.2. | There is a goods lift exclusive for the use of gluten-free food. | |||||||
| 1.7. | Toilets and dressing rooms for employees | |||||||
| 1.7.1. | Toilets equipped with washbasins and products intended for personal hygiene: antiseptic odorless liquid soap or odorless liquid soap and antiseptic, non-recycled paper towel or other safe and hygienic drying system, collectors with lid and without manual activation. | |||||||
| 1.8. | Washbasins in the production area | |||||||
| 1.8.1. | Existence of washbasins in the production area with running water, in appropriate positions in relation to the production and service flow, with sufficient number to suit the entire production area, preferably equipped with automatic stopcock, antiseptic odorless liquid soap or odorless liquid soap and antiseptic, non-recycled paper towels or other hygienic and safe drying system and paper collectors without manual activation. | |||||||
| 1.9. | Ventilation and air conditioning | |||||||
| 1.9.1. | Artificially air-conditioned environments, without fans, without generating airflow and absence of natural airflow from the production area of gluten-containing food to the production area of gluten-free food, avoiding an environment with particles in suspension. | |||||||
| 1.10. | Cleaning of the facilities | |||||||
| 1.10.1. | Facilities kept under appropriate hygienic-sanitary conditions, that is, without the presence of accumulation of residues, with proof by means of registration in specific spreadsheets, updated and with information consistent with what is being observed. | |||||||
| 1.10.2. | Utensils used for the cleaning of facilities distinct from those used for the cleaning of equipment that come into contact with food, with hygiene products and utensils exclusive for the use in the production area of gluten-free food. | |||||||
| 1.11. | Waste management | |||||||
| 1.11.1. | Containers for the collection of waste inside the establishment which are easily sanitized (i.e., without cracks that allow dirt to accumulate and are difficult to access by cleaning utensils) and transported (i.e., can be easily moved by those responsible for the procedure); emptied whenever its content reaches 2/3 of its capacity and constantly sanitized, showing no evidence of accumulated dirt; use of appropriate garbage bags. | |||||||
| 1.11.2. | Waste removed from the gluten-containing food production area does not pass through the production area of gluten-free food. | |||||||
| 1.12. | Layout | |||||||
| 1.12.1. | Layout suitable for the productive process: number, capacity, and distribution of dependencies according to the branch of activity, production volume, and expedition. | |||||||
| 1.12.2. | Areas for receiving and depositing ingredients distinct from the areas of production, storage, and expedition of the final product. | |||||||
| 1.12.3. | Gluten-free ingredients warehouse identified and in a different space from that of gluten-containing ingredients. | |||||||
| 1.12.4. | Area of production of gluten-free food identified and in a separate space from that of the production area of gluten-containing food. | |||||||
| 2. | Equipment, furniture, and kitchenware | |||||||
| 2.1. | Equipment | |||||||
| 2.1.1. | Equipment arranged in a way that allows easy access and proper cleaning. | |||||||
| 2.1.2. | Equipment with contact surfaces which are smooth, undamaged, waterproof, and easy to clean. | |||||||
| 2.1.3. | Production line equipment (mixers, processors, blenders, toasters, etc.) identified and exclusive to the production of gluten-free food. | |||||||
| 2.1.4. | Food preservation equipment (refrigerators, freezers, cold rooms) exclusive for gluten-free products or, when not possible, the disposal of products is done in separate spots and/or with some kind of physical separation between gluten-free and gluten-containing products. | |||||||
| 2.1.5. | Thermal processing equipment (ovens) exclusive for gluten-free food or, when of common use, not used for baking gluten-free and gluten-containing food simultaneously. | |||||||
| 2.1.6. | Thermal processing equipment (fryers, hot plate for tapiocas, pancakes, and others) exclusive for gluten-free food. | |||||||
| 2.2. | Furniture (tables, workbenches, window displays, shelves) | |||||||
| 2.2.1. | Furniture designed for easy cleaning (smooth, without wrinkles and cracks, and of a waterproof material). | |||||||
| 2.2.2. | Existence of specific furniture for the production of gluten-free food or existence of a proper cleaning process between the use of the furniture for gluten-containing and gluten-free food proved by an updated registration worksheet with information consistent with what is being observed. | |||||||
| 2.3. | Kitchenware | |||||||
| 2.3.1. | Kitchenware of material, size, and shape that allow easy cleaning. | |||||||
| 2.3.2. | General kitchenware (pans, spoons, knives, cutlery, etc.) exclusive for gluten-free food, stored in an appropriate and identified place, in organized manner, and protected against contamination by gluten or, when not exclusive, properly sanitized prior to the usage and preparation of gluten-free food. | |||||||
| 2.3.3. | Difficult to clean kitchenware (sieves, pastry brush, graters, etc.) exclusive for the production of gluten-free food. | |||||||
| 2.4. | Cleaning of equipment, machinery, furniture, and kitchenware | |||||||
| 2.4.1. | Equipment, machinery, furniture, and kitchenware kept in proper hygienic-sanitary conditions, that is, without the presence or accumulation of residues, with proof by means of registration in specific spreadsheets, updated, and with information consistent with what is observed. | |||||||
| 2.4.2. | Availability of cleaning products required to perform the operation and dilution, contact time, and form of use/application according to the instructions recommended by the manufacturer. | |||||||
| 2.4.3. | Availability and suitability of all necessary utensils to carry out the cleaning operation with those in good condition. | |||||||
| 2.4.4. | Whenever gluten-free food is handled, cleaning of equipment, machinery, furniture, and kitchenware that are of common use for gluten-free and gluten-containing foods is performed properly. | |||||||
| 2.4.5. | Use of an exclusive sponge or similar to sanitize all kitchenware, equipment, and surfaces that will come into contact with gluten-free food. | |||||||
| 2.4.6. | Dishwasher usage: crockery used for gluten-containing and gluten-free food sanitized at different moments. | |||||||
| 3. | Food service employees | |||||||
| 3.1. | Clothing | |||||||
| 3.1.1. | Employees display proper personal cleanliness: body cleanliness, clean hands, short nails, clean uniforms. | |||||||
| 3.1.2. | Employees use a uniform exclusive for handling gluten-free food or a uniform which has not been previously used to handle food with gluten, without having been washed afterwards. | |||||||
| 3.2. | Hygienic habits | |||||||
| 3.2.1. | There is guidance (posters) for proper hand hygiene, which includes appropriate moments and procedures, accessible to employees and followed correctly. | |||||||
| 3.2.2. | Employees do not handle gluten-containing and gluten-free foods simultaneously or engage in any act that could lead to cross-contamination, such as eating during food preparation. | |||||||
| 3.3. | Employees training program and supervision | |||||||
| 3.3.1. | Existence of a proper and continuous training program related to the production of gluten-free food and registration of these trainings. | |||||||
| 3.3.2. | Existence of supervision of the procedures to avoid gluten contamination by a properly trained supervisor. | |||||||
| 4. | Food production and transport | |||||||
| 4.1. | Raw materials, ingredients, and package | |||||||
| 4.1.1. | Raw materials, ingredients, and packaging are inspected at the reception, observing if the labels of the raw material and ingredients meet the specific legislation for gluten. Potential sources of gluten are identified and controlled upon reception. | |||||||
| 4.1.2. | Defrosting of gluten-free food held in a separate location from gluten-containing food and without getting in touch with utensils and equipment where gluten-containing food is stored or held in locations that are cleaned before procedure. | |||||||
| 4.2. | Selection of recipes and ingredients and food preparation | |||||||
| 4.2.1. | The selection of recipes and ingredients and the manufacturing technical cards are accurately followed for gluten-free food, with the label of all ingredients being checked at the time of preparation. | |||||||
| 4.2.2. | Water or oil previously used in the preparation of gluten-containing food is not reused at the preparation of gluten-free food. | |||||||
| 4.2.3. | Ingredients are not of common use for the production of gluten-free and gluten-containing food (e.g., margarine). All products intended for the preparation of gluten-free food are identified. | |||||||
| 4.3. | Production flow | |||||||
| 4.3.1. | The reception of gluten-free products occurs in a separated space from other products or carried out at a different moment. | |||||||
| 4.3.2. | Segregation or separation of procedures such as production scheduling or specific/exclusive lines for gluten-free food, with an ordered flow without crossing between gluten-free and gluten-containing food. | |||||||
| 4.4. | Labeling and storage of final product and/or semi-prepared products | |||||||
| 4.4.1. | Final and/or semi-finished products (products that will be used in the elaboration of pasta, fillings, sauces, etc.), packaged in a suitable container (known composition of the container material—gluten-free), intact and exclusive for gluten-free food. | |||||||
| 4.4.2. | Labeling statements with visible identification and in accordance with current legislation regarding the presence or absence of gluten. | |||||||
| 4.4.3. | Products with and without gluten stored separately by a physical barrier or proper distance, in order to avoid contact between them. | |||||||
| 4.5. | Transportation of the final product | |||||||
| 4.5.1. | Transportation maintains the integrity of food. | |||||||
| 4.5.2. | The vehicle does not simultaneously carry gluten-containing and gluten-free food or it does carry these products simultaneously, but with due care of separation by physical barrier or proper distance between them (use of sealed containers, of impermeable material). | |||||||
| 5. | Distribution | |||||||
| 5.1. | At the distribution of food, employees follow procedures to eliminate the risk of gluten contamination, through hand hygiene, use of protective utensils, and disposable gloves and others whenever there is previous contact with gluten-containing food. | |||||||
| 5.2. | Separate disposal, at different distribution counters. Preparation according to the presence/absence of gluten. | |||||||
| 5.3. | Preparation identified with labels or other visible method according to its gluten content. | |||||||
| 5.4. | Kitchenware used for serving food exclusive for gluten-free preparation and identified with different colors. | |||||||
| 5.5. | Monitoring of the preparation identification plates in regards to the presence/absence of gluten at the moment of distribution. | |||||||
| 6. | Documentation | |||||||
| 6.1. | Manual of good practices | |||||||
| 6.1.1. | Operations carried out at the facility are in accordance with an on-site Good Practices Manual that meets the legal requirements in regards to content and updating. | |||||||
| 6.2. | Proper hygienization of furniture and facilities in order to prevent gluten contamination | |||||||
| 6.2.1. | Existence of Standard operating procedures established for this item, which are being fulfilled. | |||||||
| 6.3. | Proper hygienization of surfaces, equipment, and kitchenware in order to prevent gluten contamination | |||||||
| 6.3.1. | Existence of SOPs established for this item, which are being fulfilled. | |||||||
| 6.4. | Food recall program | |||||||
| 6.4.1. | Existence of SOPs established for this item, which is being fulfilled. | |||||||
| 7. | Responsibility and authority | |||||||
| 7.1. | Responsibilities and authorities are defined and communicated within the organization to ensure effective operation and maintenance of the gluten contamination control. | |||||||
| 8. | Coordinator of the food safety team | |||||||
| 8.1. | Top management has a Gluten Contamination Control Team Coordinator. | |||||||
| 8.2. | The designated Coordinator has the responsibility and authority to administer the Gluten Contamination Control Team and to organize their work. | |||||||
| 8.3. | The designated Coordinator has the responsibility and authority to ensure relevant training and education of all members of the gluten contamination control team. | |||||||
| 8.4. | The designated Coordinator has the responsibility and authority to ensure that the gluten contamination control system is established, implemented, maintained, and updated. | |||||||
| 9. | Methods for comunication in the gluten contamination control | |||||||
| 9.1. | The organization ensures that the team is informed in proper time of changes of raw materials, ingredients, and services. | |||||||
| 9.2. | The organization ensures that the team is informed in proper time of changes in production systems and equipment. | |||||||
| 9.3. | The organization ensures that the team is informed in proper time of changes in production facilities, location of equipment, and surroundings. | |||||||
| 9.4. | The organization ensures that the team is informed in proper time of changes in cleaning and sanitation programs. | |||||||
| 9.5. | The organization ensures that the team is informed in proper time of changes in levels of staff qualification and/or designation of responsibilities and authorities. | |||||||
| 9.6. | The organization ensures that the team is informed in proper time of changes in knowledge regarding gluten contamination and control measures. | |||||||
| 9.9. | The organization ensures that the team is informed as soon as possible in the event of a consumer complaint indicating a possible risk of gluten contamination in the food. | |||||||
| 9.10. | The organization ensures that the team is informed in proper time of any circumstances or occurrences not covered in the previous items that may have an impact on the control of gluten contamination. | |||||||
| 9.11. | The team ensures that any information relevant to the control of gluten contamination is always updated in the system by the responsible party and passed on to the rest of the employees. | |||||||
| 10. | Flow charts | |||||||
| 10.1. | Flowcharts are prepared for categories of products or processes (implemented) by the gluten contamination control system. | |||||||
| 10.2. | Flowcharts are clear, precise, and sufficiently detailed. | |||||||
| 10.3. | Flowcharts are checked on site by the gluten contamination control team and verification records are kept. | |||||||
| 11. | Treatment of potentially unsafe products | |||||||
| 11.1. | The organization treats non-compliant products preventing them from entering the food production chain or attesting the presence of gluten on the label of such foods in case of possible contamination. | |||||||
| 11.2. | All food produced that may have been affected by a nonconformity situation is kept under the control of the organization until it has been evaluated. | |||||||
| 11.3. | The organization notifies interested parties when products that are no longer under the organization’s control are subsequently determined to be unsafe (contaminated with gluten), initiating the recall process. | |||||||
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C. Gluten sensitivity. Ann. Nutr. Metab. 2015, 67 (Suppl. 2), 16–26. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Draft Revised Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten, Joint FAO/WHO Food Standards Program, 30ty Session, ALINORM08/31/26 Appendix III; Food and Agriculture Organization/World Health Organization: Geneva, Switerland, 2008. [Google Scholar]
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Farage, P.; Zandonadi, R.P. The Gluten-Free Diet: Difficulties Celiac Disease Patients have to Face Daily. Austin J. Nutri. Food Sci. 2014, 2, 1–8. [Google Scholar]
- Araújo, H.M.C.; Araújo, W.M.C.; Botelho, R.B.A.; Zandonadi, R.P. Doença celíaca, hábitos e práticas alimentares e qualidade de vida. Rev. Nutr. 2010, 23, 467–474. [Google Scholar] [CrossRef]
- Diaz-Amigo, C.; Popping, B. Gluten and gluten-free: Issues and considerations of labeling regulations, detection methods, and assay validation. J. AOAC Int. 2012, 95, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.M.V.; Zandonadi, R.P.; Gandolfi, L.; de Almeida, R.C.; Almeida, L.M.; Pratesi, R. Evaluation of the presence of gluten in beans served at self-service restaurants: A problem for celiac disease carriers. J. Culin. Sci. Technol. 2014, 12, 22–33. [Google Scholar] [CrossRef]
- Silva, R.P.; Lordello, M.L.L.; Nishitokukado, I.; Ortiz-Agostinho, C.L.; Santos, F.M.; Leite, A.Z.; Sipahi, A.M. Detection and quantification of gluten in processed food by ELISA in Brazil. Gastroenterology 2010, 138, S306. [Google Scholar]
- Laureano, A.M. Análise da Presença de Glúten em Alimentos Rotulados como Livres de Glúten Através de Ensaio Imunoenzimático e de Fitas Imunocromatográficas. Master’s Thesis, Federal University of Rio Grande do Sul, Porto Alegre, Brazil, 23 May 2010. [Google Scholar]
- Farage, P.; de Medeiros Nóbrega, Y.K.; Pratesi, R.; Gandolfi, L.; Assunção, P.; Zandonadi, R.P. Gluten contamination in gluten-free bakery products: A risk for coeliac disease patients. Public Health Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, C.; Naspi, G.; Catassi, C. Health-related quality of life in children with celiac disease: A study based on the critical incident technique. Nutrients 2013, 5, 4476–4485. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, A.; Foglini, M.; Paolini, F.; Framboas, M.; Serena Altissimi, M.; Naceur Haouet, M.; Mangili, P.; Osimani, A.; Clementi, F.; Cenci, T.; et al. Evaluation of the quality of foods for special diets produced in a school catering facility within a HACCP-based approach: A case study. Int. J. Environ. Health Res. 2013, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bicudo, M.O.P. Avaliação da Presença de Glúten em Produtos Panificados para Celíacos—Estudo de caso. Master’s Thesis, Federal University of Paraná, Curitiba, Brazil, 2010. [Google Scholar]
- Health Canada. Health Canada’s Position on Gluten-Free Claims. 2012. Available online: http://www.hc-sc.gc.ca/fn-an/securit/allerg/cel-coe/gluten-position-eng.php (accessed on 10 February 2016). [Google Scholar]
- Código Alimentario Argentino. Resolución Conjunta 131/2011. Available online: http://www.alimentosargentinos.gob.ar/contenido/marco/CAA/ModificacionesCAA.html (accessed on 10 February 2016).
- Lima, T.C. Content validation of an instrument to characterize people over 50 years of age living with Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome. Acta Paul. Enfer. 2012, 25, 4–10. [Google Scholar] [CrossRef]
- Polit, D.F.; Beck, C.T. Nursing Research: Principles and Methods, 7th ed.; Lippincott Willians and Wilkings: Philadelphia, PA, USA, 2004. [Google Scholar]
- Wendisch, C. Avaliação da Qualidade de Unidades de Alimentação e Nutrição (UAN) Hospitalares: Construção de um Instrumento. Master’s Thesis, Osvaldo Cruz Foundation, Sergio Arouca National School of Public Health, Rio de Janeiro, Brazil, 10 October 2010. [Google Scholar]
- Conti, M.A.; Scagliusi, F.; de Oliveira Queiroz, G.K.; Hearst, N.; Cordás, T.A. Adaptação transcultural: tradução e validação de conteúdo para o idioma português do modelo da Tripartite Influence Scale de insatisfação corporal. Cad Saúde Pública 2010, 26, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC n° 216, de 15 de Setembro de 2004; Diário Oficial da República Federativa do Brasil: Brasília, Brazil, 2004.
- Brasil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC n° 275, de 21 de Outubro de 2002; Diário Oficial da República Federativa do Brasil: Brasília, Brazil, 2003.
- International Organization for Standardization (ISO). ISO 22000. Food Safety Management Systems and Requirements for Any Organization in the Food Chain; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Canadian Celiac Association. Standards and Policies Respecting the Certification of Gluten-Free Products under the Gluten-Free Certification Program; Canadian Celiac Association: Mississauga, ON, Canada, 2011. [Google Scholar]
- Hollon, J.R.; Cureton, P.A.; Martin, M.L.; Leonard Puppa, E.L.; Fasano, A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ceniccola, G.D.; Araújo, W.M.C.; Akutsu, R. Development of a tool for quality control audits in hospital enteral nutrition. Nutr. Hosp. 2014, 29, 102–120. [Google Scholar]
- Reichenheim, M.E.; Moraes, C.L. Operacionalização de adaptação transcultural de instrumentos de aferição usados em epidemiologia. Rev. Saúde Pública 2007, 41, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, L. Testes referentes ao construto: Teoria e modelo da construção. In Instrumentos Psicológicos: Manual Prático de Elaboração; Labpam: Brasília, Brazil, 1999. [Google Scholar]
- Pasquali, L. Psicometria. Rev. Esc. Enferm. USP 2009, 43, 992–999. [Google Scholar] [CrossRef]
- Alexandre, N.M.C.; Coluci, M.Z.O. Validade de conteúdo nos processos de construção e adaptação de instrumentos de medidas. Ciência Saúde Coletiva 2011, 16, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Saccol, A.L.F.; Stangarlin, L.; Hecktheuer, L.H. Instrumentos de Apoio para Implantação das boas Práticas em Empresas Alimentícias, 1st ed.; Editora Rubio: Rio de Janeiro, Brasil, 2012. [Google Scholar]
- Araújo, H.M.C.; Araújo, W.M.C. Coeliac disease. Following the diet and eating habits of participating individuals in the Federal District, Brazil. Appetite 2011, 57, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Karajeh, M.A.; Hurlstone, D.P.; Patel, T.M.; Sanders, D.S. Chefs’ knowledge of coeliac disease (compared to the public): A questionnaire survey from the United Kingdom. Clin. Nutr. 2005, 24, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Laporte, L.; Zandonadi, R.P. Conhecimento dos chefes de cozinha acerca da doença celíaca. Aliment. Nutr. 2011, 22, 465–470. [Google Scholar]
- Machado, J.; Ganolfi, L.; de Almeida, R.C.; Almeida, L.M.; Zandonadi, R.P.; Pratesi, R. Gluten-free dietary compliance in Brazilian celiac patients: questionnaire versus serological test. Nutr. Clín. Diet. Hosp. 2013, 33, 46–49. [Google Scholar]
| Section of the Check-List | Content Validation (Mean Grade ± SD *) | Content Validation (W-Value) | Semantic Evaluation (Mean Grade ± SD *) | Semantic Evaluation (W-Value) |
|---|---|---|---|---|
| Building and facilities | 4.74 ± 0.30 | 0.96 | 4.76 ± 0.15 | 0.92 |
| Equipment, furniture and kitchenware | 4.79 ± 0.25 | 0.97 | 4.83 ± 0.17 | 0.96 |
| Food service employees | 4.81 ± 0.20 | 0.98 | 4.79 ± 0.20 | 0.93 |
| Food production and transport | 4.79 ± 0.21 | 0.96 | 4.87 ± 0.17 | 0.98 |
| Distribution | 4.86 ± 0.14 | 0.94 | 5.00 ± 0.00 | 1.00 |
| Documentation | 4.82 ± 0.27 | 0.96 | 4.75 ± 0.32 | 0.96 |
| Responsibility and authority | 4.86 ± 0.00 | 1.00 | 5.00 ± 0.00 | 1.00 |
| Coordinator of the food security team | 4.57 ± 0.26 | 0.89 | 5.00 ± 0.00 | 1.00 |
| Internal communication | 4.78 ± 0.23 | 0.92 | 4.71 ± 0.29 | 0.94 |
| Flow charts | 4.86 ± 0.14 | 1.00 | 4.71 ± 0.14 | 0.90 |
| Treatment of potentially unsafe products | 4.52 ± 0.24 | 0.90 | 4.76 ± 0.20 | 0.95 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farage, P.; Puppin Zandonadi, R.; Cortez Ginani, V.; Gandolfi, L.; Pratesi, R.; De Medeiros Nóbrega, Y.K. Content Validation and Semantic Evaluation of a Check-List Elaborated for the Prevention of Gluten Cross-Contamination in Food Services. Nutrients 2017, 9, 36. https://doi.org/10.3390/nu9010036
Farage P, Puppin Zandonadi R, Cortez Ginani V, Gandolfi L, Pratesi R, De Medeiros Nóbrega YK. Content Validation and Semantic Evaluation of a Check-List Elaborated for the Prevention of Gluten Cross-Contamination in Food Services. Nutrients. 2017; 9(1):36. https://doi.org/10.3390/nu9010036
Chicago/Turabian StyleFarage, Priscila, Renata Puppin Zandonadi, Verônica Cortez Ginani, Lenora Gandolfi, Riccardo Pratesi, and Yanna Karla De Medeiros Nóbrega. 2017. "Content Validation and Semantic Evaluation of a Check-List Elaborated for the Prevention of Gluten Cross-Contamination in Food Services" Nutrients 9, no. 1: 36. https://doi.org/10.3390/nu9010036






