Evidence for Involvement of IL-9 and IL-22 in Cows’ Milk Allergy in Infants
Abstract
:1. Introduction
2. Materials and Methods
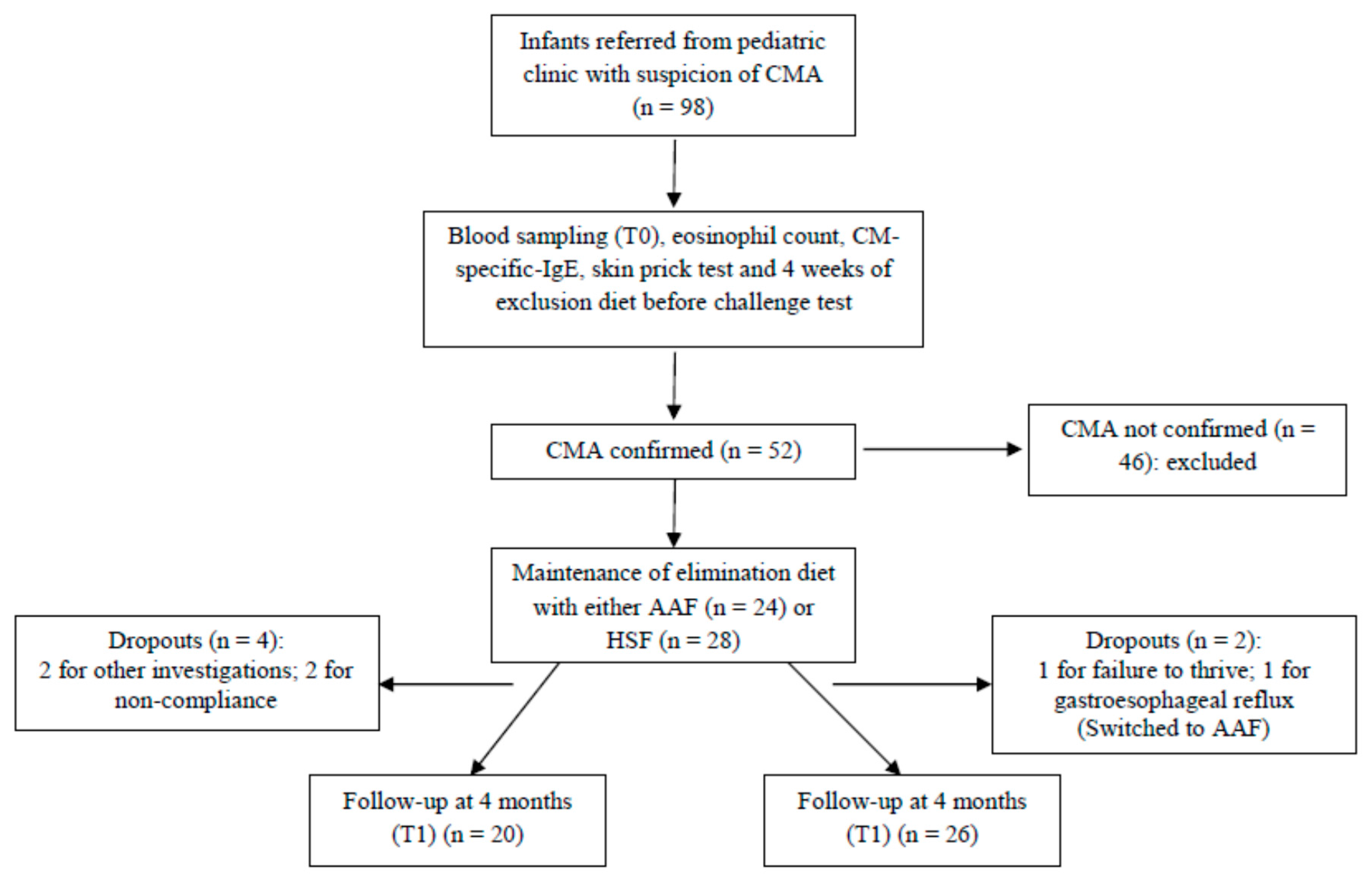
2.1. Participants and Study Design
2.2. Open Oral Challenge Test with Cows’ Milk
2.3. Blood Tests
2.4. Skin Prick Tests
2.5. Plasma Cytokine Concentrations
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Effect of Dietary Treatment on Plasma Cytokine Concentrations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAF | free amino acid formula |
| BF | breast fed |
| CM | cows’ milk |
| CMA | cows’ milk allergy |
| HSF | hydrolysed soy protein formula |
| IFN | interferon |
| IgE | immunoglobulin E |
| IL | interleukin |
| SPT | skin prick test |
| TNF | tumor necrosis factor |
References
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; EAACI food allergy and anaphylaxis guidelines group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Available online: https://www.nice.org.uk/guidance/qs118 (accessed on 6 September 2017).
- Tordesillas, L.; Berin, M.C.; Sampson, H.A. Immunology of food allergy. Immunity 2017, 47, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Immunologic influences on allergy and the TH1/TH2 balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Tupa, D.; Graham, M.T.; Chatila, T.A.; Spergel, J.M.; Nadeau, K.C. Deciphering the black box of food allergy mechanisms. Ann. Allergy Asthma Immunol. 2017, 118, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S.; Annunziato, F. Th17 and non-classic Th1 cells in chronic inflammatory disorders: Two sides of the same coin. Int. Arch. Allergy Immunol. 2014, 164, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Berker, M.; Frank, L.J.; Geßner, A.L.; Grassl, N.; Holtermann, A.V.; Höppner, S.; Kraef, C.; Leclaire, M.D.; Maier, P.; Messerer, D.A.; et al. Allergies—A T cells perspective in the era beyond the TH1/TH2 paradigm. Clin. Immunol. 2017, 174, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ. J. 2010, 3, 57–161. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T.; Standards of Care Committee (SOCC) of the British Society for Allergy and Clinical Immunology (BSACI). BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Abuabat, A.; Al-Hammadi, S.; Aly, G.S.; Miqdady, M.S.; Shaaban, S.Y.; Torbey, P.H. Middle East consensus statement on the prevention, diagnosis, and management of cow’s milk protein allergy. Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Souwer, Y.; Szegedi, K.; Kapsenberg, M.L.; de Jong, E.C. IL-17 and IL-22 in atopic allergic disease. Curr. Opin. Immunol. 2010, 22, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The role of il-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- Farfariello, V.; Amantini, C.; Nabissi, M.; Morelli, M.B.; Aperio, C.; Caprodossi, S.; Carlucci, A.; Bianchi, A.M.; Santoni, G. IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitis and asthmatic pediatric patients. Pediatr. Allergy Immunol. 2011, 22, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Stassen, M.; Schmitt, E.; Bopp, T. From interleukin-9 to T helper 9 cells. Ann. N. Y. Acad. Sci. 2012, 1247, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Dardalhon, V.A.; Awasthi, H.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-b-induced Foxp3+ T cells and, together with TGF-b, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Uyttenhove, C.; Van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9–Producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Bindslev-Jensen, C.; Ballmer-Weber, B.K.; Bengtsson, U.; Blanco, C.; Ebner, C.; Hourihane, J.; Knulst, A.C.; Moneret-Vautrin, D.A.; Nekam, K.; Niggemann, B.; et al. European Academy of Allergology and Clinical Immunology. Standardization of food challenges in patients with immediate reactions to foods—Position paper from the European Academy of Allergology and Clinical Immunology. Allergy 2004, 59, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Niggermenn, B.; Beyer, K. Diagnosis of food allergy in children: Toward a standardization of food challenge. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.T.; Matsul, E.C.; Conover-Walker, M.K.; Wood, R.A. Risk of oral food challenges. J. Allergy Clin. Immunol. 2004, 114, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A. Update on food allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Assa’ad, A.H.; Bahna, S.L.; Bock, A.S.; Sicherer, S.H.; Teuber, S.S.; Adverse Reactions to Food Committee of American, Academy of Allergy, Asthma & Immunology. Work Group Report: Oral food challenge testing. J. Allergy Clin. Immunol. 2009, 123, S365–S383. [Google Scholar] [CrossRef] [PubMed]
- Schade, R.P.; Van Leperen-Van Dijk, A.G.; Van Reijsen, F.C.; Versluis, C.; Kimpen, J.L.; Knol, E.F.; Bruijnzeel-Koomen, C.A.; Van Hoffen, E. Differences in antigen-specific T-cell responses between infants with atopic dermatitis with and without cow’s milk allergy: Relevance of Th2 cytokines. J. Allergy Clin. Immunol. 2000, 106, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Goto, Y.; Miike, T. Markedly high eosinophilia and an elevated serum IL-5 level in an infant with cow milk allergy. Ann. Allergy Asthma Immunol. 1999, 82, 253–256. [Google Scholar] [CrossRef]
- Sonnenberg, G.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 increases the innate immunity of tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Nair, M.G.; Kim, T.J.; Zaph, C.; Fouser, L.A.; Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010, 207, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Temann, U.A.; Geba, G.P.; Rankin, J.A.; Flavell, R.A. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 1998, 188, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Noh, G.; Lee, S.J.; Lee, J.H.; Kim, H.S.; Choi, W.S. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell. Immunol. 2012, 273, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.B.; Hvid, M.; Kvist, P.H.; Deleuran, B.; Deleuran, M.; Vestergaard, C.; Kemp, K. CD4+ T cell depletion changes the cytokine environment from a TH1/TH2 response to a TC17-like response in a murine model of atopic dermatitis. Int. Immunopharmacol. 2011, 9, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, M.M.; Lehtonen, O.P.; Eerola, E.; Kero, P. Fecal microflora in healthy infants born by different methods of delivery: Permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Miniello, V.L.; Colasanto, A.; Cristofori, F.; Diaferio, L.; Ficele, L.; Lieggi, M.S.; Santoiemma, V.; Francavilla, R. Gut microbiota biomodulators, when the stork comes by the scalpel. Clin. Chim. Acta. 2015, 451, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, M.M.; Arvilommi, H.; Kero, P.; Lehtonen, O.P.; Isolauri, E. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: A prospective follow up study of healthy infants aged 0-6 months. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 83, F186–F192. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Wohlfahrt, J.; Westergaard, T. Caesarean delivery and risk of atopy and allergic disease: Meta-analyses. Clin. Exp. Allergy 2008, 38, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, C.; Manti, S.; Salpietro, A.; Alterio, T.; Arrigo, T.; Leonardi, S.; Salpietro, C. Mode of delivery and risk for development of atopic diseases in children. Allergy Asthma Proc. 2015, 36, 344–351. [Google Scholar] [CrossRef] [PubMed]
- West, C.E. Gut microbiota and allergic disease: New findings. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 261–266. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | HSF | AAF | BF |
|---|---|---|---|
| Number | 26 | 20 | 11 |
| Sex: | |||
| Female, n (%) | 9 (36) | 11 (52) | 5 (45) |
| Male, n (%) | 17 (64) | 9 (48) | 6 (55) |
| Mean age at T0 (months) | 8.0 ± 0.8 | 5.6 ± 0.6 | - |
| (range) | 2–17 | 2–13 | |
| Mean age at T1 (months) | 12.0 ± 0.8 | 9.4 ± 0.6 | 12.5 ± 1.8 |
| (range) | 6–21 | 6–17 | 8–25 |
| Mode of birth: | |||
| Cesarean, n (%) | 23 (89) ** | 19 (97) ** | 3 (27) |
| Vaginal, n (%) | 3 (11) ** | 1 (3) ** | 8 (73) |
| Birth weight (g) | 3122 ± 56 | 3115 ± 41 | 3158 ± 88 |
| Length at birth (cm) | 47.9 ± 0.4 | 47.9 ± 0.9 | 47.8 ± 0.6 |
| Exclusive breastfeeding (months) | 2.7 ± 0.5 * | 2.4 ± 0.4 * | 4.9 ± 0.3 |
| Maternal age (years) | 29.3 ± 1.5 | 30.5 ± 1.7 | 26.3 ± 0.9 |
| Maternal education: | |||
| High school, n (%) | 8 (30) ** | 8 (40) ** | 100 (11) |
| College, n (%) | 19 (70) ** | 12 (60) ** | 0 (0) |
| Clinical Description | HSF (n = 26) | AAF (n = 20) |
|---|---|---|
| Type of allergy: | ||
| Immunoglobulin (IgE) mediated | 7 (27) | 3 (15) |
| Non-IgE mediated | 15 (58) | 16 (80) |
| Mixed | 4 (15) | 1 (5) |
| Clinical symptoms: | ||
| Gastrointestinal | ||
| Diarrhea/constipation/colic | 11 (42) | 5 (20) |
| Vomiting/regurgitation/reflux | 7 (27) | 5 (25) |
| Colitis/blood in stools | 13 (50) | 13 (65) |
| Respiratory | ||
| Wheeze | 6 (23) | 1 (5) |
| Skin | ||
| Contact urticaria | 11 (42) | 4 (20) |
| Atopic dermatitis | 0 (0) | 1 (5) |
| Systemic | ||
| Anaphylaxis | 1 (4) | 1 (5) |
| Failure to thrive | 0 (0) | 4 (20) |
| Marker of Allergy | Infants with CMA (n = 46) |
|---|---|
| ImmunoCap negative, % (n) | 39 (18) |
| Blood eosinophils (cells/mm3) * | 232.3 (72.0–693.9) |
| Total IgE (IU/mL) * | 8.55 (2.3–195.0) |
| Cows’ milk protein-specific IgE (IU/mL) * | 1.94 (0.04–77.64) |
| Anti-alpha lactalbumin IgE (IU/mL) * | 1.47 (0.08–35.08) |
| Anti-beta lactalbumin IgE (IU/mL) * | 1.38 (2.98–25.0) |
| Anti-casein IgE (IU/mL) * | 1.12 (0.002–39.36) |
| Anti-soya IgE (IU/mL) * | 0.0 (0.0–3.52) |
| Cytokine | CMA (T0) | CMA (T1) | P for CMA (T0) vs. CMA (T1) | BF | P for CMAT0 vs. BF * |
|---|---|---|---|---|---|
| IL-1β | 2.0 (2.0–48.0) | 2.0 (2.0–15.5) | 0.132 | 2.0 (2.0–13.3) | 0.324 |
| IL-2 | 10.0 (10.0–71.56) | 39.9 (10.0–65.6) | 0.948 | 17.8 (7.7–92.9) | 0.498 |
| IL-4 | 35.9 (10.0–159.9) | 10.0 (10.0–60.9) | 0.001 | 10.0 (8.7–55.6) | 0.022 |
| IL-9 | 1.0 (1.0–20.3) | 1.0 (1.0–7.5) | 0.003 | 2.7 (1.2–5.8) | 0.038 |
| IL-12p70 | 1.0 (1.0–19.1) | 1.0 (1.0–8.7) | 0.083 | 1.0 (1.0–14.3) | 0.905 |
| IL-13 | 48.4 (3.0–58.7) | 13.0 (3.0–55.5) | 0.001 | 3.8 (2.2–20.2) | <0.01 |
| IL-17A | 1.50 (1.50–84.5) | 10.5 (1.5–40.6) | 0.002 | 6.9 (1.0–21.3) | 0.039 |
| IL-22 | 145.7 (20.0–278.5) | 103.2 (20.0–176.8) | 0.018 | 109.5 (20.0–166.8) | 0.128 |
| TNF-α | 4.9 (1.4–58.8) | 3.4 (1.0–46.7) | 0.247 | 8.1 (1.3–29.8) | 0.229 |
| IFN-γ | 1.0 (1.0–45.9) | 1.0 (1.0–85.6) | 0.138 | 5.8 (1.0–81.9) | 0.033 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, K.V.; Flor Silveira, V.L.; Laranjeira, M.S.; Wandalsen, N.F.; Passeti, S.; De Oliveira, R.; Munekata, R.V.; Noakes, P.S.; Miles, E.A.; Calder, P.C. Evidence for Involvement of IL-9 and IL-22 in Cows’ Milk Allergy in Infants. Nutrients 2017, 9, 1048. https://doi.org/10.3390/nu9101048
Barros KV, Flor Silveira VL, Laranjeira MS, Wandalsen NF, Passeti S, De Oliveira R, Munekata RV, Noakes PS, Miles EA, Calder PC. Evidence for Involvement of IL-9 and IL-22 in Cows’ Milk Allergy in Infants. Nutrients. 2017; 9(10):1048. https://doi.org/10.3390/nu9101048
Chicago/Turabian StyleBarros, Karina V., Vera L. Flor Silveira, Marisa S. Laranjeira, Neusa F. Wandalsen, Susana Passeti, Roberta De Oliveira, Regina V. Munekata, Paul S. Noakes, Elizabeth A. Miles, and Philip C. Calder. 2017. "Evidence for Involvement of IL-9 and IL-22 in Cows’ Milk Allergy in Infants" Nutrients 9, no. 10: 1048. https://doi.org/10.3390/nu9101048




