Phyllodulcin, a Natural Sweetener, Regulates Obesity-Related Metabolic Changes and Fat Browning-Related Genes of Subcutaneous White Adipose Tissue in High-Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Animals and Diet
2.3. Biochemical Analysis of Blood Samples
2.4. Western Blotting Analysis
2.5. RNA Isolation and Real-Time Polymerase Chain Reaction (PCR) Analysis
2.6. Statistical Analyses
3. Results
3.1. B.W., Food Intake, and Fat and Liver Weight
3.2. Plasma Biochemical Profiles
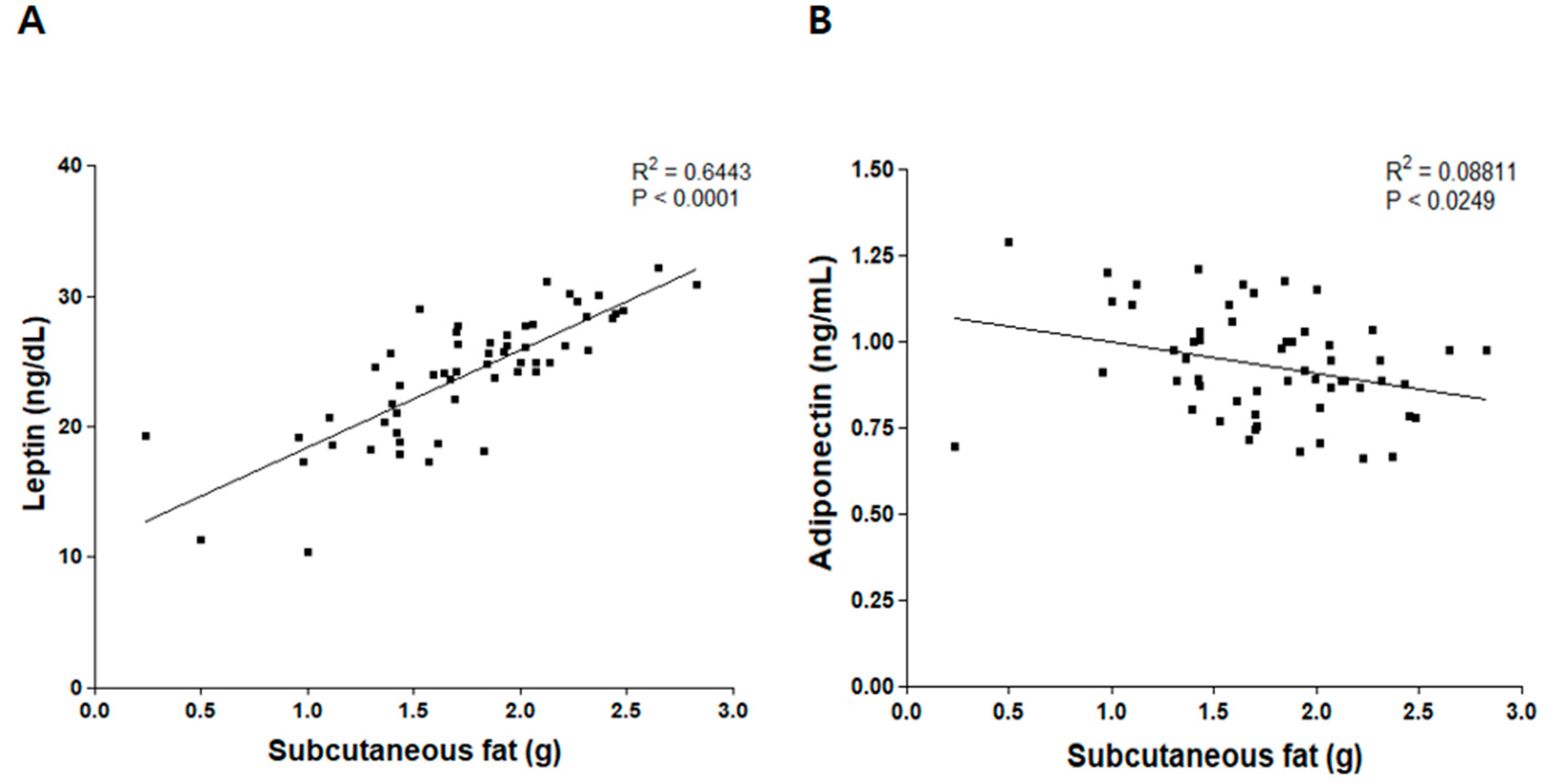
3.3. Correlation between Weight of Subcutaneous Fat and Levels of Leptin and Adiponectin
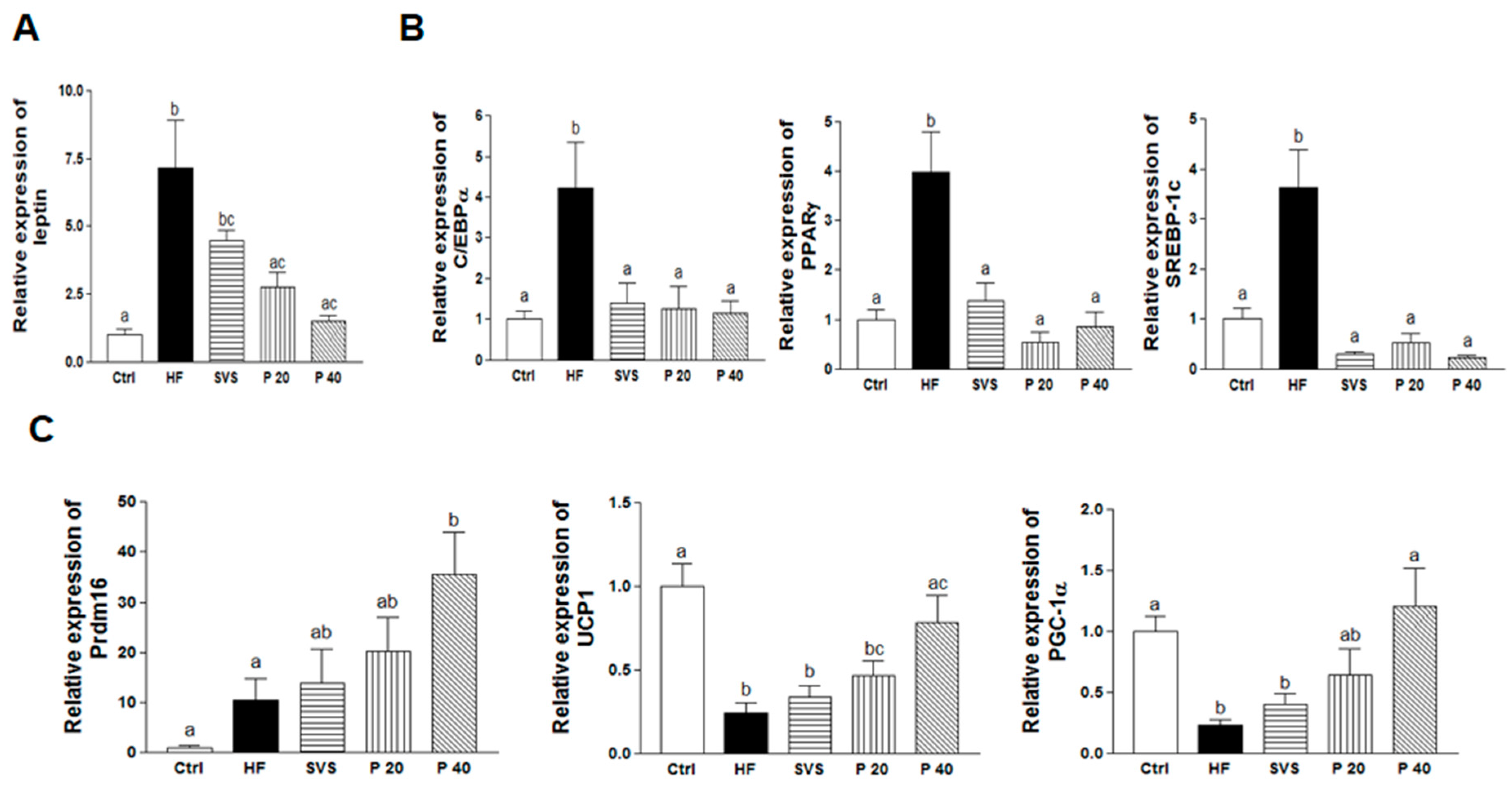
3.4. Expression of Genes Related to Adipogenesis, Lipogenesis, and Browning in Subcutaneous Fat
3.5. Brain Weight and BDNF Signaling in Hypothalamus
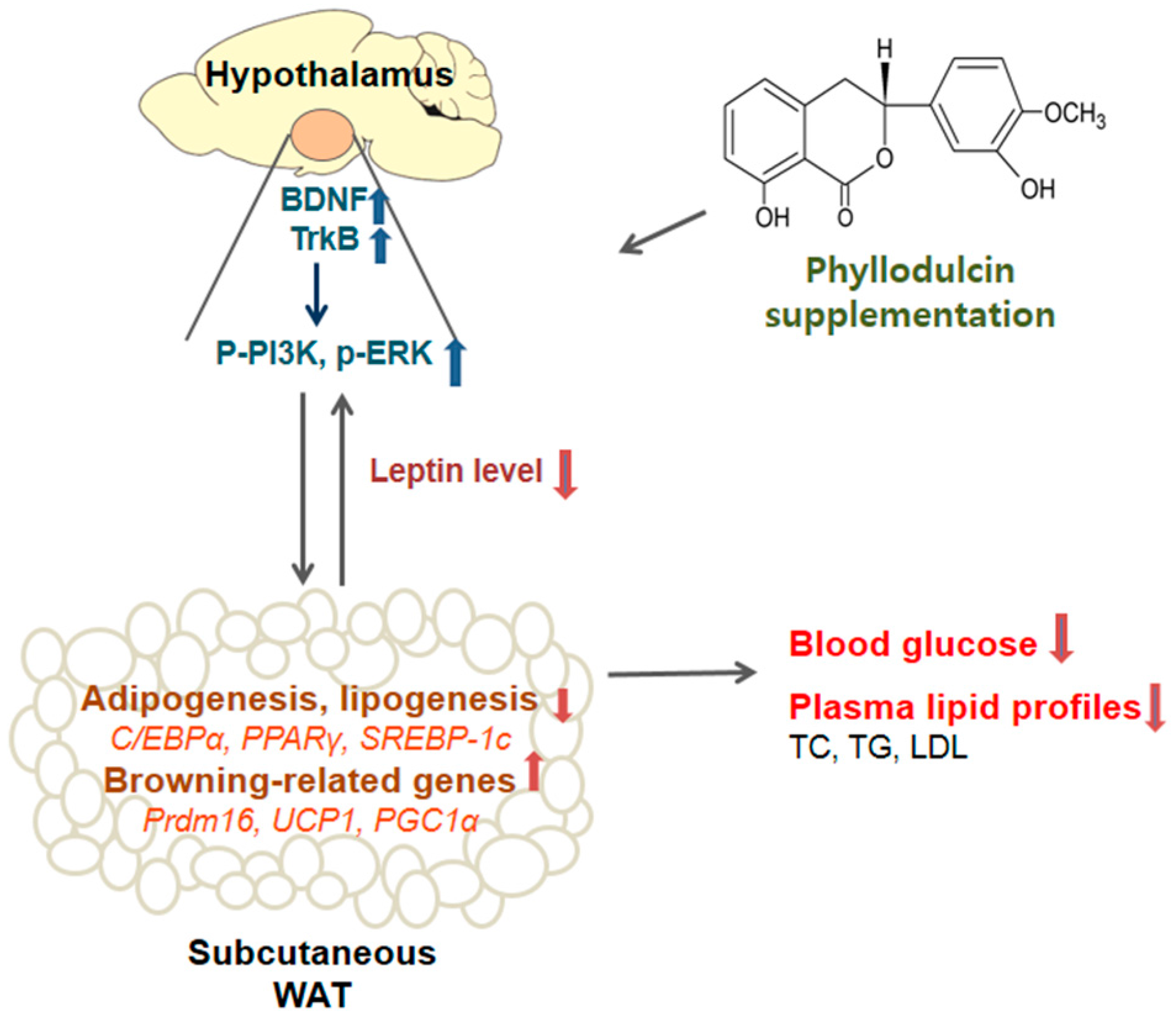
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Fact Sheet: Obesity and Overweight; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Rui, L. Brain regulation of energy balance and body weight. Rev. Endocr. Metab. Disord. 2013, 14, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.A.; Ertel, N.; Schneider, G. Obesity, hormones, and cancer. Cancer Res. 1981, 41, 3711–3717. [Google Scholar] [PubMed]
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10 (Suppl. 2), 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottiere, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Adipocyte differentiation and transdifferentiation: Plasticity of the adipose organ. J. Endocrinol. Investig. 2002, 25, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, J.; Cereijo, R.; Villarroya, F. An endocrine role for brown adipose tissue? Am. J. Physiol. Endocrinol. Metab. 2013, 305, E567–E572. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef] [PubMed]
- Hesselbarth, N.; Pettinelli, C.; Gericke, M.; Berger, C.; Kunath, A.; Stumvoll, M.; Bluher, M.; Kloting, N. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in c57bl/6ntac mice. Biochem. Biophys. Res. Commun. 2015, 464, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Mattson, M.P. Bdnf mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Vanevski, F.; Xu, B. Molecular and neural bases underlying roles of bdnf in the control of body weight. Front. Neurosci. 2013, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Y.; An, J.J.; Gharami, K.; Waterhouse, E.G.; Vanevski, F.; Jones, K.R.; Xu, B. Dendritically targeted bdnf mrna is essential for energy balance and response to leptin. Nat. Med. 2012, 18, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in c57bl/6j mice. Int. J. Obes. 2000, 24, 639–646. [Google Scholar] [CrossRef]
- Kievit, P.; Howard, J.K.; Badman, M.K.; Balthasar, N.; Coppari, R.; Mori, H.; Lee, C.E.; Elmquist, J.K.; Yoshimura, A.; Flier, J.S. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in pomc-expressing cells. Cell Metab. 2006, 4, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A. The real contribution of added sugars and fats to obesity. Epidemiol. Rev. 2007, 29, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Mitsutomi, K.; Masaki, T.; Shimasaki, T.; Gotoh, K.; Chiba, S.; Kakuma, T.; Shibata, H. Effects of a nonnutritive sweetener on body adiposity and energy metabolism in mice with diet-induced obesity. Metabolism 2014, 63, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Kayaba, S.; Takahashi, K.; Nakazawa, T.; Ohsawa, K. Metabolic fate of orally administered phyllodulcin in rats. J. Nat. Prod. 2004, 67, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Matsuda, H.; Kumahara, A.; Ito, Y.; Nakamura, S.; Yoshikawa, M. New type of anti-diabetic compounds from the processed leaves of hydrangea macrophylla var. Thunbergii (hydrangeae dulcis folium). Bioorg. Med. Chem. Lett. 2007, 17, 4972–4976. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Yamada, M.; Tsuda, Y.; Kawai, K.; Nakajima, S. Antifungal activity of oosponol, oospolactone, phyllodulcin, hydrangenol, and some other related compounds. Chem. Pharm. Bull. (Tokyo) 1981, 29, 2689–2691. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Hashigaki, K.; Uenishi, J.; Yamakawa, I.; Sato, N. Chemical structure and sweet taste of isocoumarin and related compounds. Vi. Chem. Pharm. Bull. 1975, 23, 3101–3105. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Hashigaki, K.; Mito, K.; Koyama, T. Chemical structure and sweet taste of isocoumarins and related compounds. X. Syntheses of sweet 5-hydroxyflavanones and related dihydrochalcones. Chem. Pharm. Bull. 1978, 26, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Kagata, M.; Masaki, E.; Nishi, H. Phyllodulcin, a constituent of “amacha”, inhibits phosphodiesterase in bovine adrenocortical cells. Pharmacol. Toxicol. 2002, 90, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Matsuda, H.; Yamahara, J.; Yoshikawa, M. Development of bioactive functions in hydrangeae dulcis folium. Vii. Immunomodulatory activities of thunberginol a and related compounds on lymphocyte proliferation. Biol. Pharm. Bull. 1998, 21, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kim, Y.; Kim, M.S.; Lee, S.; Yoo, S.H. The establishment of efficient bioconversion, extraction, and isolation processes for the production of phyllodulcin, a potential high intensity sweetener, from sweet hydrangea leaves (hydrangea macrophylla thunbergii). Phytochem. Anal. 2016, 27, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Lim, J.Y.; Shin, J.H.; Seok, P.R.; Jung, S.; Yoo, S.H.; Kim, Y. D-xylose suppresses adipogenesis and regulates lipid metabolism genes in high-fat diet-induced obese mice. Nutr. Res. 2015, 35, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Hube, F.; Lietz, U.; Igel, M.; Jensen, P.B.; Tornqvist, H.; Joost, H.G.; Hauner, H. Difference in leptin mrna levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm. Metab. Res. 1996, 28, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Vijay, V.; Satyavani, K.; Latha, E.; Haffner, S.M. Plasma leptin in non-diabetic asian indians: Association with abdominal adiposity. Diabet. Med. 1997, 14, 937–941. [Google Scholar] [CrossRef]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Blackburn, G.L.; Kanders, B.S.; Lavin, P.T.; Keller, S.D.; Whatley, J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am. J. Clin. Nutr. 1997, 65, 409–418. [Google Scholar] [PubMed]
- Geeraert, B.; Crombe, F.; Hulsmans, M.; Benhabiles, N.; Geuns, J.; Holvoet, P. Stevioside inhibits atherosclerosis by improving insulin signaling and antioxidant defense in obese insulin-resistant mice. Int. J. Obes. 2010, 34, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, L.; Guo, C.; Han, B.; Pan, C.; Zhao, S.; Song, H.; Ma, Q. Stevioside ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by downregulating the nf-κb pathway. Biochem. Biophys. Res. Commun. 2012, 417, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, A.; Gavrilovic, M.; Jakovljevic, V.; Sabo, J. Glucose concentration in the blood of intact and alloxan-treated mice after pretreatment with commercial preparations of stevia rebaudiana (bertoni). Eur. J. Drug Metab. Pharm. 2004, 29, 87–90. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Campfield, L.A.; Smith, F.J.; Guisez, Y.; Devos, R.; Burn, P. Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science 1995, 269, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.-I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Matsui, T.; Kawachi, H.; Yamada, T.; Nakanishi, N.; Yano, H. Fat depot-specific differences in leptin mrna expression and its relation to adipocyte size in steers. Anim. Sci. J. 2003, 74, 17–21. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T. Dietary n-3 fatty acids affect mrna level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000, 84, 175–184. [Google Scholar] [PubMed]
- Krempler, F.; Breban, D.; Oberkofler, H.; Esterbauer, H.; Hell, E.; Paulweber, B.; Patsch, W. Leptin, peroxisome proliferator-activated receptor-γ, and ccaat/enhancer binding protein-α mrna expression in adipose tissue of humans and their relation to cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Gollisch, K.S.; Brandauer, J.; Jessen, N.; Toyoda, T.; Nayer, A.; Hirshman, M.F.; Goodyear, L.J. Effects of exercise training on subcutaneous and visceral adipose tissue in normal-and high-fat diet-fed rats. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E495–E504. [Google Scholar] [CrossRef] [PubMed]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Montague, C.T.; Prins, J.B.; Sanders, L.; Digby, J.E.; O’rahilly, S. Depot-and sex-specific differences in human leptin mrna expression: Implications for the control of regional fat distribution. Diabetes 1997, 46, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Coulter, A.; Rim, J.S.; Koza, R.A.; Kozak, L.P. Transcriptional synergy and the regulation of ucp1 during brown adipocyte induction in white fat depots. Mol. Cell. Biol. 2005, 25, 8311–8322. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Plutzky, J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab. J. 2016, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Münzberg, H.; Zhang, Z.-Y.; Kahn, B.B. Leptin and insulin act on pomc neurons to promote the browning of white fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Gu, P.; Zhang, J.; Nie, T.; Pan, Y.; Wu, D.; Feng, T.; Zhong, C.; Wang, Y.; Lam, K.S.; et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting m2 macrophage proliferation. Cell Metab. 2015, 22, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [PubMed]
- Tontonoz, P.; Kim, J.B.; Graves, R.A.; Spiegelman, B.M. Add1: A novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 1993, 13, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Tontonoz, P.; Spiegelman, B.M. Transdifferentiation of myoblasts by the adipogenic transcription factors ppar gamma and c/ebp alpha. Proc. Natl. Acad. Sci. USA 1995, 92, 9856–9860. [Google Scholar] [CrossRef] [PubMed]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. Srebp transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Gomez-Ambrosi, J.; Martin, M.; Moncada, R.; Sesma, P.; Burrell, M.A.; Fruhbeck, G. Role of prdm16 in the activation of brown fat programming. Relevance to the development of obesity. Histol. Histopathol. 2013, 28, 1411–1425. [Google Scholar] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of prdm16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.A.; Sun, L. Turning wat into bat: A review on regulators controlling the browning of white adipocytes. Biosci. Rep. 2013, 33, e00065. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by prdm16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, A.; Stadler, U.; Glotzer, M.A.; Kozak, L.P. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mrna expression. J. Biol. Chem. 1985, 260, 16250–16254. [Google Scholar] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the pgc-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H. Prdm16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a prdm16–c/ebp-β transcriptional complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Quan, J.I. From white to brown fat through the pgc-1alpha-dependent myokine irisin: Implications for diabetes and obesity. Dis. Model. Mech. 2012, 5, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Sidossis, L.; Kajimura, S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Jequier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E. Neurotrophism and energy homeostasis: Perfect together. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R988–R991. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, T.; Tsuchida, A.; Ono-Kishino, M.; Nakagawa, T.; Taiji, M.; Noguchia, H. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. Exp. Diabetes Res. 2001, 2, 201–209. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, R.; Yang, J.L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of pgc-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 2006, 110, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Sigmund, C.D.; Haynes, W.G.; Mark, A.L. Hypothalamic erk mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009, 58, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.; Rother, E.; Münzberg, H.; Wunderlich, F.T.; Morgan, D.A.; Hampel, B.; Shanabrough, M.; Janoschek, R.; Könner, A.C.; Alber, J. Enhanced leptin-stimulated pi3k activation in the cns promotes white adipose tissue transdifferentiation. Cell Metab. 2007, 6, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Palou, A.; Bonet, M.L. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J. Nutr. Biochem. 2011, 22, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Yang, J.Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3t3-l1 adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Park, Y.; Gonzalez, F.J.; Pariza, M.W. Influence of conjugated linoleic acid on body composition and target gene expression in peroxisome proliferator-activated receptor α-null mice. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2001, 1533, 233–242. [Google Scholar] [CrossRef]

| Gene Symbol | Genbank ID | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | |
|---|---|---|---|---|
| Leptin | Lep | 16486 | TGACACCAAAACCCTCATCA | CTCAAAGCCACCACCTCTGT |
| C/EBPα | Cebpa | 12606 | CCAAGAAGTCGGTGGACAAGA | CGGTCATTGTCACTGGTCAACT |
| PPARγ | Pparg | 19016 | AAGAGCTGACCCAATGGTTG | TGAGGCCTGTTGTAGAGCTG |
| SREBP-1c | Srebf1 | 20787 | TAGAGCATATCCCCCAGGTG | GGTACGGGCCACAAGAAGTA |
| Prdm16 | Prdm16 | 70673 | AGATGAACCAGGCATCCACT | TCTACGTCCTCTGGCTTTGC |
| UCP1 | UCP1 | 22227 | CCAAGCCAGGATGGTGAAC | CCAGCGGGAAGGTGATGATA |
| PGC-1α | Ppargc1a | 19017 | TCGAGCTGTACTTTTGTGGA | TCATACTTGCTCTTGGTGGA |
| GAPDH | Gapdh | 14433 | GCCTTCCGTGTTCCTACCC | TGCCTGCTTCACCACCTT |
| Ctrl | HF | SVS | P 20 | P 40 | |
|---|---|---|---|---|---|
| Final b.w. (g) | 35.56 ± 0.98 a | 43.73 ± 0.88 b | 43.58 ± 1.62 b | 43.59 ± 0.79 b | 43.95 ± 1.05 b |
| BMI (kg/m2) | 5.82 ± 0.18 a | 6.90 ± 0.14 b | 6.76 ± 0.14 b | 6.62 ± 0.26 b | 6.32 ± 0.10 ab |
| Food intake (g/d) | 3.23 ± 0.02 a | 2.92 ± 0.03 b | 2.68 ± 0.41 c | 2.81 ± 0.03 b | 2.83 ± 0.03 b |
| Total fat mass of b.w. (%) | 11.69 ± 0.77 a | 14.93 ± 0.39 b | 14.22 ± 0.40 b | 13.30 ± 0.99 ab | 13.24 ± 0.27 ab |
| Subcutaneous fat (g) | 1.16 ± 0.11 a | 2.11 ± 0.07 b | 1.88 ± 0.09 bc | 1.88 ± 0.20 bc | 1.63 ± 0.08 c |
| Mesenteric fat (g) | 0.67 ± 0.06 a | 1.21 ± 0.08 b | 1.32 ± 0.06 b | 1.15 ± 0.13 b | 1.17 ± 0.08 b |
| Ctrl | HF | SVS | P 20 | P 40 | |
|---|---|---|---|---|---|
| TG (mg/dL) | 107.32 ± 5.51 a | 121.62 ± 4.91 b | 116.26 ± 3.10 ab | 104.68 ± 1.60 a | 103.76 ± 1.68 a |
| TC (mg/dL) | 106.87 ± 5.92 a | 132.89 ± 3.15 b | 113.46 ± 4.96 a | 113.00 ± 0.03 a | 113.66 ± 2.17 a |
| LDL (mg/dL) | 34.14 ± 3.64 a | 48.33 ± 2.70 b | 29.46 ± 2.71 a | 29.78 ± 5.19 a | 27.91 ± 3.15 a |
| FBG (mg/dL) | 111.30 ± 6.88 a | 208.18 ± 10.87 b | 137.50 ± 6.22 ac | 138.58 ± 5.63c | 131.17 ± 3.54 ac |
| Leptin (ng/mL) | 18.18 ± 1.31 a | 27.47 ± 0.50 b | 25.95 ± 0.92 ab | 24.81 ± 1.54 ab | 22.63 ± 0.79 c |
| Adiponectin (ng/mL) | 1.10 ± 0.04 a | 0.81 ± 0.04 b | 0.82 ± 0.02 bc | 0.95 ± 0.04 cd | 1.00 ± 0.03 ad |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Lim, S.-M.; Kim, M.-S.; Yoo, S.-H.; Kim, Y. Phyllodulcin, a Natural Sweetener, Regulates Obesity-Related Metabolic Changes and Fat Browning-Related Genes of Subcutaneous White Adipose Tissue in High-Fat Diet-Induced Obese Mice. Nutrients 2017, 9, 1049. https://doi.org/10.3390/nu9101049
Kim E, Lim S-M, Kim M-S, Yoo S-H, Kim Y. Phyllodulcin, a Natural Sweetener, Regulates Obesity-Related Metabolic Changes and Fat Browning-Related Genes of Subcutaneous White Adipose Tissue in High-Fat Diet-Induced Obese Mice. Nutrients. 2017; 9(10):1049. https://doi.org/10.3390/nu9101049
Chicago/Turabian StyleKim, Eunju, Soo-Min Lim, Min-Soo Kim, Sang-Ho Yoo, and Yuri Kim. 2017. "Phyllodulcin, a Natural Sweetener, Regulates Obesity-Related Metabolic Changes and Fat Browning-Related Genes of Subcutaneous White Adipose Tissue in High-Fat Diet-Induced Obese Mice" Nutrients 9, no. 10: 1049. https://doi.org/10.3390/nu9101049




