A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: a Prospective, Randomized, Controlled Trial
Abstract
:1. Introduction
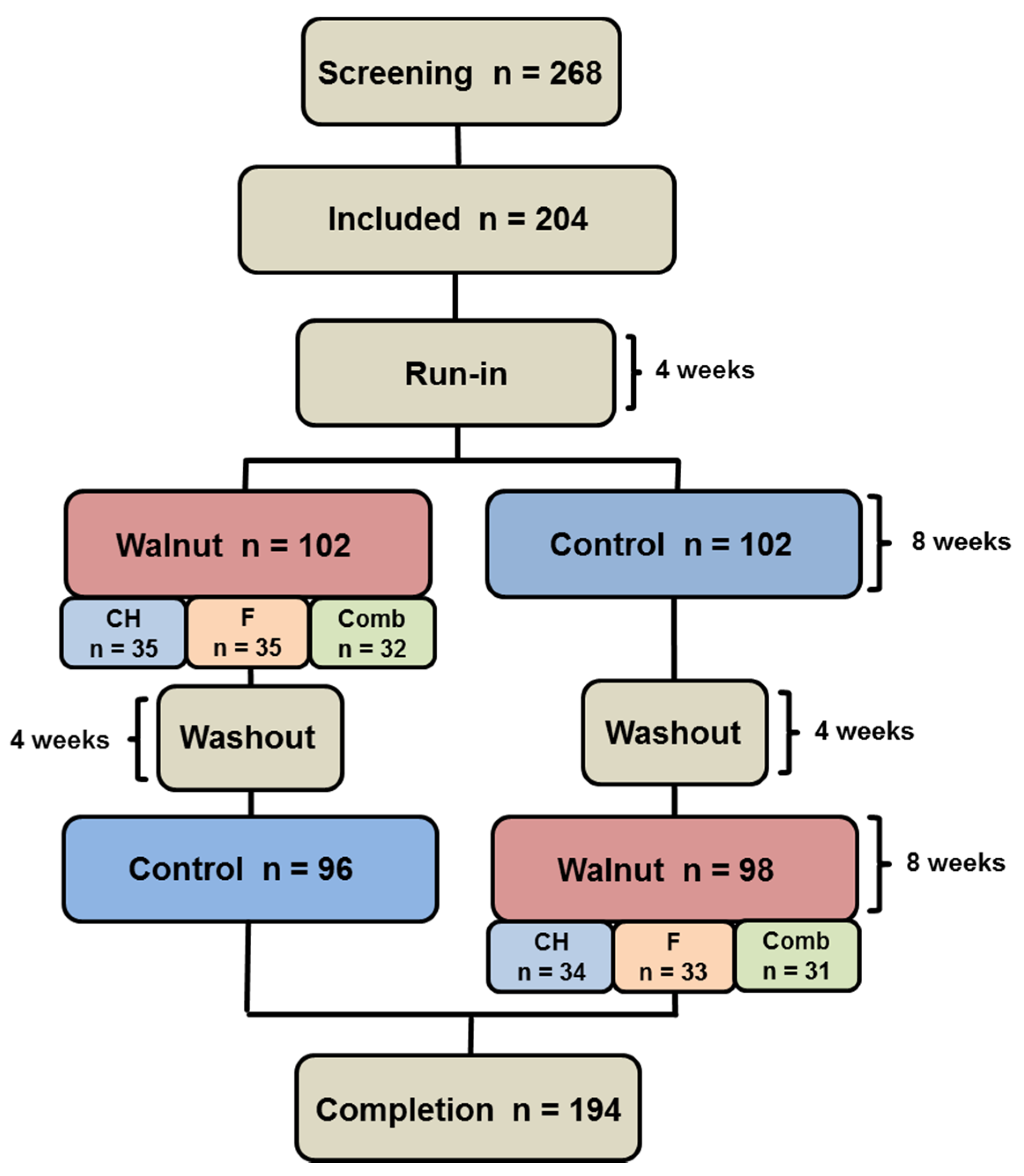
2. Subjects and Methods
2.1. Subjects
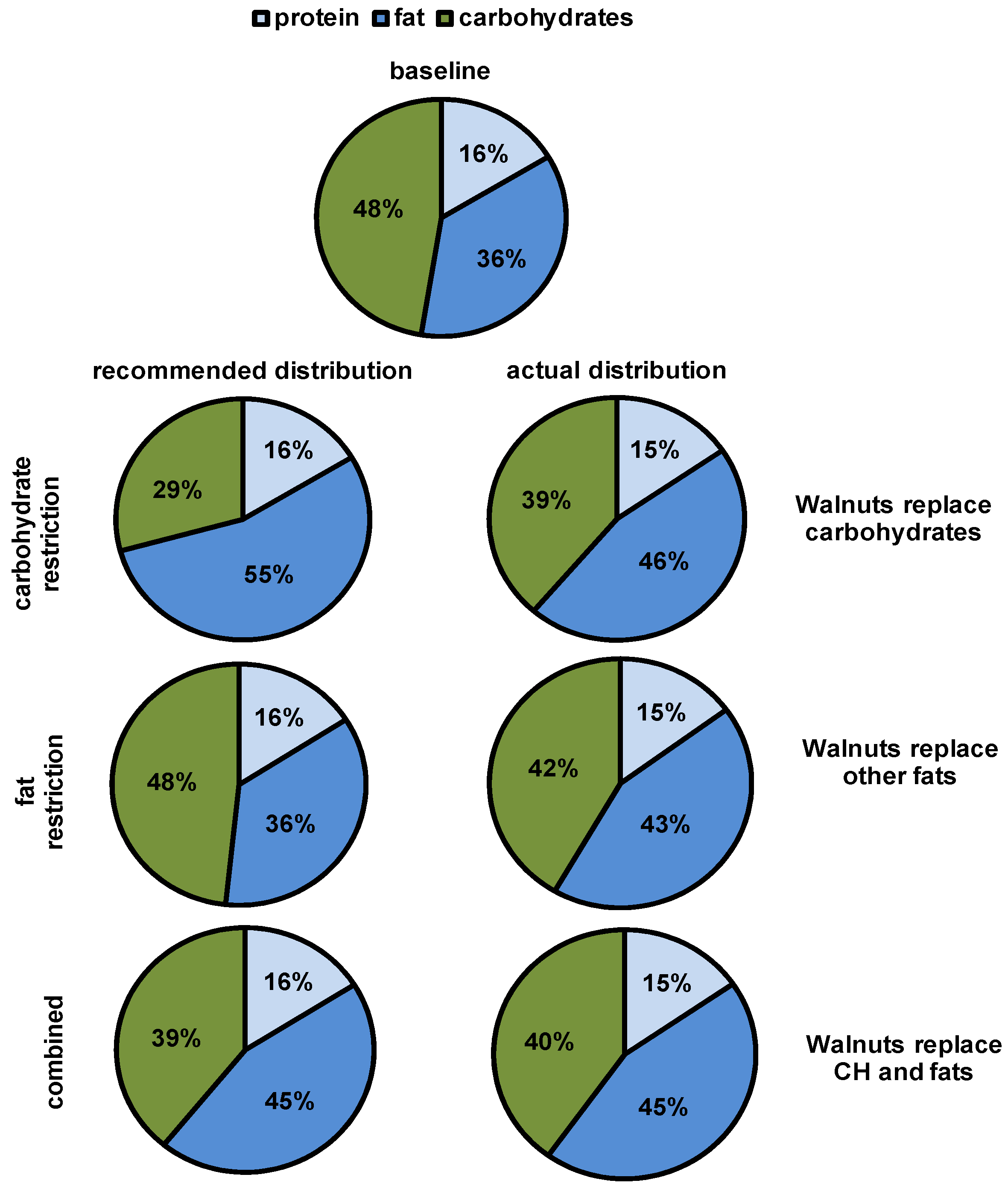
2.2. Study Design
2.3. Laboratory Measurements
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
4.1. Lipid-Lowering Effect
4.2. Macronutrient Replaced and Time Point of Consumption
4.3. Other Findings
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| apoB | apolipoprotein B |
| BMI | body mass index |
| CH | carbohydrates |
| comb | combined |
| CVD | cardiovascular disease |
| ET-1 | endothelin 1 |
| F | fat |
| HbA1c | hemoglobin A1c |
| HDL-C | high-density lipoprotein cholesterol |
| hsCRP | high-sensitive C-reactive protein |
| LDL-C | low-density lipoprotein cholesterol |
| Lp (a) | lipoprotein a |
| n-3 | omega-3 |
| PUFA | polyunsaturated fatty acids |
| TG | triglycerides |
| TC | total cholesterol |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VLDL-C | very-low-density lipoprotein cholesterol |
References
- Schaefer, E.J. Lipoproteins, nutrition, and heart disease. Am. J. Clin. Nutr. 2002, 75, 191–212. [Google Scholar] [PubMed]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, CD004816. [Google Scholar] [CrossRef]
- Mannu, G.S.; Zaman, M.J.; Gupta, A.; Rehman, H.U.; Myint, P.K. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr. Cardiol. Rev. 2013, 9, 2–14. [Google Scholar] [PubMed]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Gordon, R.Y.; Morris, P.B.; Yorko, J.; Gordon, Y.J.; Li, M.; Iqbal, N. Simvastatin vs. therapeutic lifestyle changes and supplements: Randomized primary prevention trial. Mayo Clin. Proc. 2008, 83, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.Y.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Banel, D.K.; Hu, F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kralova Lesna, I.; Suchanek, P.; Kovar, J.; Stavek, P.; Poledne, R. Replacement of dietary saturated fas by pufas in diet and reverse cholesterol transport. J. Lipid Res. 2008, 49, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Gillen, L.J.; Patch, C.S.; Batterham, M.; Owen, A.; Bare, M.; Kennedy, M. Including walnuts in a low-fat/modified-fat diet improves hdl cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004, 27, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.; Mann, J.; Skeaff, M.; Frampton, C.; Sutherland, W.; Duncan, A.; Tiszavari, S. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur. J. Clin. Nutr. 1998, 52, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Spiller, G.A.; Jenkins, D.J.; Cragen, L.N.; Gates, J.E.; Bosello, O.; Berra, K.; Rudd, C.; Stevenson, M.; Superko, R. Effect of a diet high in monounsaturated fat from almonds on plasma cholesterol and lipoproteins. J. Am. Coll. Nutr. 1992, 11, 126–130. [Google Scholar] [PubMed]
- Cortes, B.; Nunez, I.; Cofan, M.; Gilabert, R.; Perez-Heras, A.; Casals, E.; Deulofeu, R.; Ros, E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J. Am. Coll. Cardiol. 2006, 48, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Fernandez-Ballart, J.; Ros, E.; Martinez-Gonzalez, M.A.; Fito, M.; Estruch, R.; Corella, D.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; et al. Effect of a mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the predimed randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Nunez, I.; Perez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Almario, R.U.; Vonghavaravat, V.; Wong, R.; Kasim-Karakas, S.E. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am. J. Clin. Nutr. 2001, 74, 72–79. [Google Scholar] [PubMed]
- Griel, A.E.; Kris-Etherton, P.M. Tree nuts and the lipid profile: A review of clinical studies. Br. J. Nutr. 2006, 96 (Suppl. 2), S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Piotrowski, K.; Rau, T.; Waldmann, E.; Broedl, U.C.; Demmelmair, H.; Koletzko, B.; Stark, R.G.; Nagel, J.M.; Mantzoros, C.S.; et al. Walnut-enriched diet reduces fasting non-hdl-cholesterol and apolipoprotein b in healthy caucasian subjects: A randomized controlled cross-over clinical trial. Metabolism 2014, 63, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Fraser, G.E.; Burke, K.; Knutsen, S.F.; Bennett, H.; Lindsted, K.D. Effects of walnuts on serum lipid levels and blood pressure in normal men. N. Engl. J. Med. 1993, 328, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Grundy, S.M. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. N. Engl. J. Med. 1986, 314, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Lefevre, M.; Ginsberg, H.N.; Kris-Etherton, P.M.; Elmer, P.J.; Stewart, P.W.; Ershow, A.; Pearson, T.A.; Dennis, B.H.; Roheim, P.S.; et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: Studies in the fasting and postprandial states. Am. J. Clin. Nutr. 2007, 86, 1611–1620. [Google Scholar] [PubMed]
- Hodson, L.; Skeaff, C.M.; Chisholm, W.A. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur. J. Clin. Nutr. 2001, 55, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Njike, V.Y.; Ayettey, R.; Petraro, P.; Treu, J.A.; Katz, D.L. Walnut ingestion in adults at risk for diabetes: Effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res. Care 2015, 3, e000115. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Josse, A.R.; Kendall, C.W.; Augustin, L.S.; Ellis, P.R.; Jenkins, D.J. Almonds and postprandial glycemia—A dose-response study. Metabolism 2007, 56, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Manson, J.E.; Willett, W.C.; Hu, F.B. Walnut consumption is associated with lower risk of type 2 diabetes in women. J. Nutr. 2013, 143, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D.L. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 2010, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Batterham, M.J.; Teuss, G.; Tan, S.Y.; Dalton, S.; Quick, C.J.; Gillen, L.J.; Charlton, K.E. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur. J. Clin. Nutr. 2009, 63, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Moloney, F.; Toomey, S.; Noone, E.; Nugent, A.; Allan, B.; Loscher, C.E.; Roche, H.M. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes 2007, 56, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Sharp, S.J.; Burgess, S.; Perry, J.R.; Stewart, I.D.; Willems, S.M.; Luan, J.; Ardanaz, E.; Arriola, L.; Balkau, B.; et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: A meta-analysis. JAMA 2016, 316, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Quon, M.J.; Han, S.H.; Lee, Y.; Kim, S.J.; Shin, E.K. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2010, 55, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Measured energy value of pistachios in the human diet. Br. J. Nutr. 2012, 107, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Dreher, M.L. Nuts and healthy body weight maintenance mechanisms. Asia Pac. J. Clin. Nutr. 2010, 19, 137–141. [Google Scholar] [PubMed]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts consumed by healthy adults provide less available energy than predicted by the atwater factors. J. Nutr. 2016, 146, 9–13. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Anthropometric Values (n = 194) | Baseline | (Min–Max) |
|---|---|---|
| Gender | m = 60 f = 134 | |
| Age (years) | 63 ± 0.54 | (50–86) |
| BMI (kg/m2) | 25.4 ± 0.29 | (17.2–35.3) |
| Waist circumference (cm) | 83.5 ± 0.81 | (62–108) |
| Fasting Metabolic Parameters | ||
| TC (mg/dL) | 231.6 ± 2.7 | (119–330) |
| Non-HDL-C (mg/dL) | 161.7 ± 2.6 | (71–243) |
| LDL-C (mg/dL) | 146.3 ± 2.3 | (54–206) |
| HDL-C (mg/dL) | 68.6 ± 1.2 | (35–111) |
| Triglycerides (mg/dL) | 101.2 ± 4.0 | (31–296) |
| Glucose (mg/dL) | 91.4 ± 0.7 | (74–118) |
| HbA1c (%) | 5.4 ± 0.02 | (4.0–6.4) |
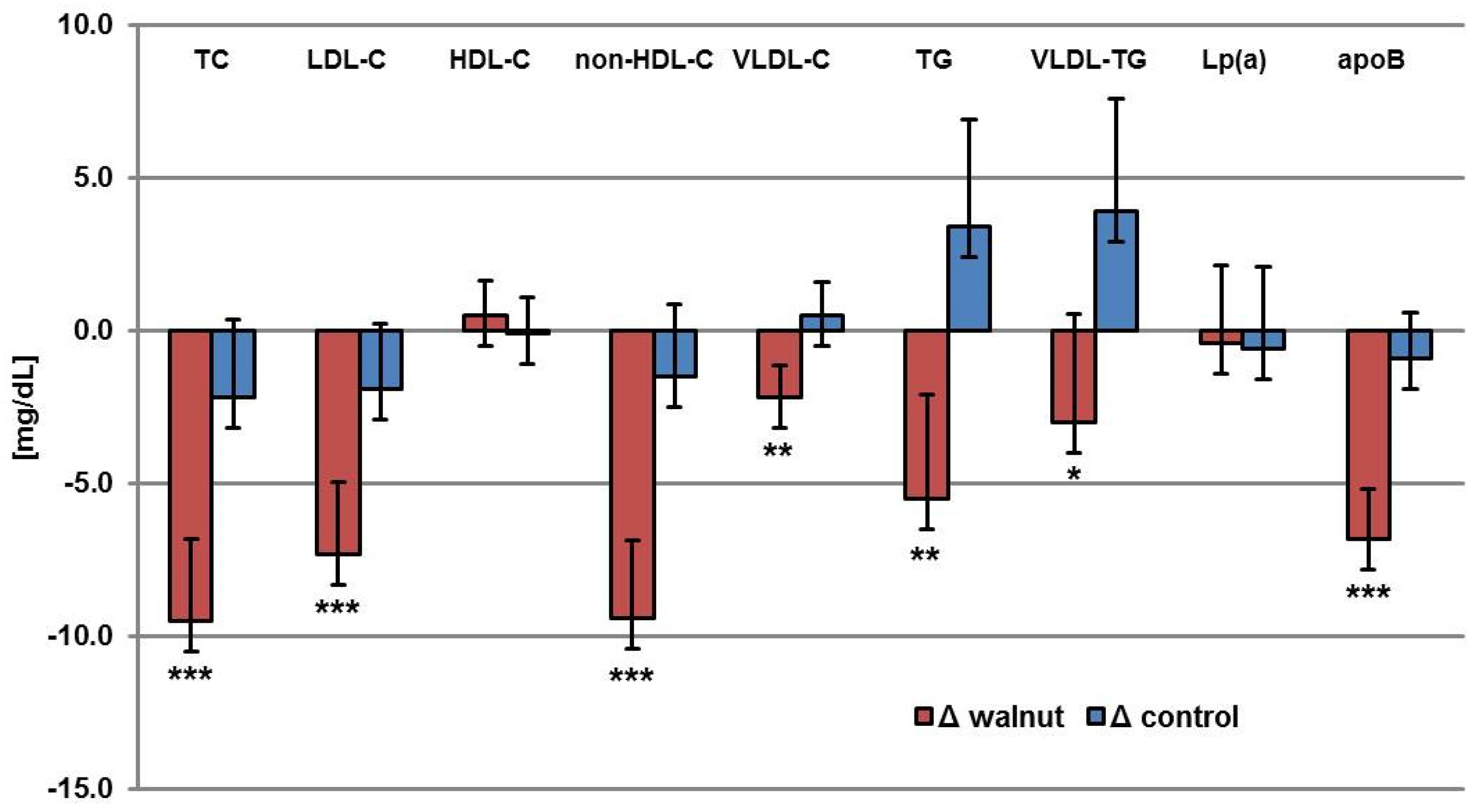
| Parameter | BaselineW | ΔWalnut | BaselineC | ΔControl | p * |
|---|---|---|---|---|---|
| TC (mg/dL) | 231.7 ± 2.7 | −9.5 | 231.6 ± 2.5 | −2.2 | 0.0003 |
| LDL-C (mg/dL) | 146.3 ± 2.3 | −7.3 | 146.0 ± 2.1 | −1.9 | 0.0009 |
| HDL-C (mg/dL) | 68.6 ± 1.1 | 0.5 | 68.8 ± 1.2 | −0.1 | 0.297 |
| non-HDL-C (mg/dL) | 163.1 ± 2.6 | −9.4 | 162.8 ± 2.4 | −1.5 | <0.0001 |
| VLDL-C (mg/dL) | 16.7 ± 1.1 | −2.2 | 16.8 ± 1.1 | 0.5 | 0.0021 |
| TG (mg/dL) | 101.2 ± 3.4 | −5.5 | 102.8 ± 3.5 | 3.4 | 0.0043 |
| VLDL-TG (mg/dL) | 74.3 ± 3.6 | −3 | 77.8 ± 3.7 | 3.9 | 0.0355 |
| Lp (a) (mg/dL) | 12 (1–139) | −0.4 | 11.5 (2–173) | −0.6 | 0.8079 |
| apoB (mg/dL) | 109.9 ± 1.6 | −6.8 | 109.5 ± 1.5 | −0.9 | <0.0001 |
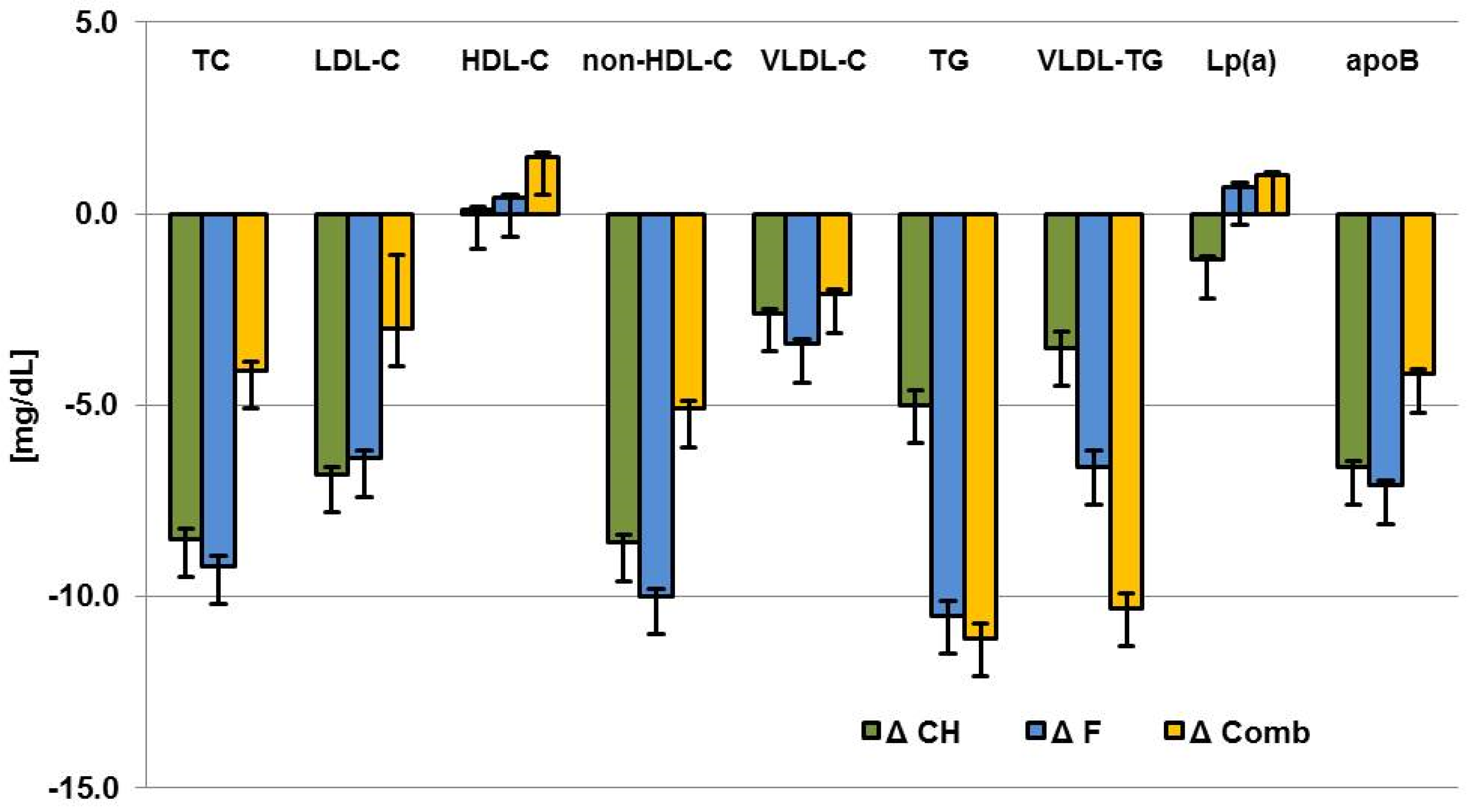
| Walnuts | Difference between Walnuts and Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | ΔCarbohydrate | ΔFat | ΔComb | p | ΔCarbohydrate | ΔFat | ΔComb | p |
| TC (mg/dL) | −11.9 ± 2.7 | −9.7 ± 2.6 | −6.2 ± 2.8 | 0.3158 | −8.5 ± 3.5 (0.0148) | −9.2 ± 3.4 (0.0070) | −4.1 ± 3.3 (0.2202) | 0.5113 |
| LDL-C (mg/dL) | −9.0 ± 2.3 | −6.9 ± 2.3 | −5.6 ± 2.4 | 0.5681 | −6.8 ± 2.8 (0.0166) | −6.4 ± 2.8 (0.0210) | −3.0 ± 2.7 (0.2677) | 0.5657 |
| HDL-C (mg/dL) | 0.1 ± 0.8 | 0.7 ± 0.8 | 1.5 ± 0.9 | 0.4817 | 0.1 ± 1.1 (0.9185) | 0.4 ± 1.1 (0.7153) | 1.5 ± 1.1 (0.1863) | 0.6688 |
| non-HDL-C (mg/dL) | −11.2 ± 2.3 | −9.6 ± 2.3 | −6.7 ± 2.4 | 0.3786 | −8.6 ± 3.1 (0.0059) | −10.0 ± 3.0 (0.0011) | −5.1 ± 3.0 (0.0886) | 0.4947 |
| VLDL-C (mg/dL) | −2.8 ± 1.1 | −2.7 ± 1.1 | −0.8 ± 1.1 | 0.3232 | −2.6 ± 1.5 (0.0871) | −3.4 ± 1.5 (0.0236) | −2.1 ± 1.5 (0.1591) | 0.8191 |
| TG (mg/dL) | −4.9 ± 3.8 | −5.5 ± 3.7 | −4.1 ± 3.9 | 0.9622 | −5.0 ± 5.5 (0.3603) | −10.5 ± 5.3 (0.0509) | −11.1 ± 5.3 (0.0371) | 0.6853 |
| VLDL-TG (mg/dL) | −1.3 ± 3.9 | −1.2 ± 3.8 | −4.1 ± 4.0 | 0.8343 | −3.5 ± 5.7 (0.5446) | −6.6 ± 5.6 (0.2383) | −10.3 ± 5.5 (0.0645) | 0.6951 |
| Lp (a) (mg/dL) | −1.4 ± 1.0 | −0.3 ± 1.0 | 0.7 ± 1.0 | 0.3405 | −1.2 ± 1.3 (0.3669) | 0.7 ± 1.3 (0.5798) | 1.0 ± 1.3 (0.4590) | 0.4471 |
| apoB (mg/dL) | −8.3 ± 1.6 | −6.5 ± 1.6 | −4.8 ± 1.6 | 0.2939 | −6.6 ± 2.1 (0.0019) | −7.1 ± 2.0 (0.0007) | −4.2 ± 2.0 (0.0392) | 0.5654 |
| Walnuts | Difference between Walnuts and Control | |||||
|---|---|---|---|---|---|---|
| Parameter | Meal | Snack | p | Meal | Snack | p |
| TC (mg/dL) | −11.6 ± 2.2 | −7.0 ± 2.3 | 0.1277 | −7.3 ± 2.7 (0.0077) | −7.1 ± 2.8 (0.0126) | 0.9515 |
| LDL-C (mg/dL) | −8.3 ± 1.9 | −6.0 ± 2.0 | 0.3948 | −5.2 ± 2.2 (0.0222) | −5.7 ± 2.3 (0.0150) | 0.8714 |
| HDL-C (mg/dL) | 0.6 ± 0.7 | 0.9 ± 0.7 | 0.7889 | 1.4 ± 0.9 (0.1058) | −0.2 ± 0.9 (0.8646) | 0.212 |
| non-HDL-C (mg/dL) | −11.3 ± 1.9 | −7.0 ± 2.0 | 0.1039 | −8.6 ± 2.4 (0.0005) | −7.1 ± 2.5 (0.0056) | 0.6538 |
| VLDL-C (mg/dL) | −3.2 ± 0.9 | −1.0 ± 0.9 | 0.0648 | −3.8 ± 1.2 (0.0017) | −1.5 ± 1.2 (0.2254) | 0.1809 |
| TG (mg/dL) | −7.5 ± 3.0 | −2.1 ± 3.2 | 0.2057 | −12.9 ± 4.3 (0.0029) | −4.7 ± 4.4 (0.2919) | 0.182 |
| VLDL-TG (mg/dL) | −4.5 ± 3.2 | −0.1 ± 3.3 | 0.2964 | −9.2 ± 4.5 (0.0427) | −4.4 ± 4.6 (0.3461) | 0.4596 |
| Lp (a) (mg/dL) | −0.3 ± 0.8 | −0.3 ± 0.8 | 0.9745 | 0.4 ± 1.1 (0.7208) | −0.02 ± 1.1 (0.9841) | 0.7927 |
| apoB (mg/dL) | −8.6 ± 1.3 | −4.5 ± 1.3 | 0.024 | −6.5 ± 1.6 (0.0001) | −5.3 ± 1.7 (0.0022) | 0.6024 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: a Prospective, Randomized, Controlled Trial. Nutrients 2017, 9, 1097. https://doi.org/10.3390/nu9101097
Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, Altenhofer J, Henze K, Parhofer KG. A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: a Prospective, Randomized, Controlled Trial. Nutrients. 2017; 9(10):1097. https://doi.org/10.3390/nu9101097
Chicago/Turabian StyleBamberger, Charlotte, Andreas Rossmeier, Katharina Lechner, Liya Wu, Elisa Waldmann, Renée G. Stark, Julia Altenhofer, Kerstin Henze, and Klaus G. Parhofer. 2017. "A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: a Prospective, Randomized, Controlled Trial" Nutrients 9, no. 10: 1097. https://doi.org/10.3390/nu9101097





