Balanced Diet-Fed Fat-1 Transgenic Mice Exhibit Lower Hindlimb Suspension-Induced Soleus Muscle Atrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
Hindlimb Suspension (HS)
2.3. Gastrocnemius Fatty Acid Composition
2.4. In Vivo Soleus Muscle Electrical Stimulation
2.5. Analysis of Soleus Muscle Force and Contractile Properties
2.6. Analysis of Soleus Muscle Resistance to Fatigue
2.7. Histological Analysis of the Soleus Muscle
2.8. Determination of Cathepsin L Activity in the Gastrocnemius Muscle
2.9. 26S Proteasome Activity Measurement in the Gastrocnemius Muscle
2.10. Analysis of Akt, S6, 4EBP1, GSK3-Beta, Atrogin-1/MAFbx, MuRF1 and ERK 1/2 in the Soleus Muscle by Western Blot
2.11. Statistical Analysis
3. Results
3.1. Body Weight Gain
3.2. Wet and Dry Mass of the Soleus, Gastrocnemius, Plantaris, Tibialis Anterior, and EDL Muscles, and Total Protein Content in the Soleus Muscle
3.3. Wet Mass of Subcutaneous, Epididymal, Retroperitoneal and Mesenteric Fat Depots
3.4. Gastrocnemius Muscle Fatty Acid Composition
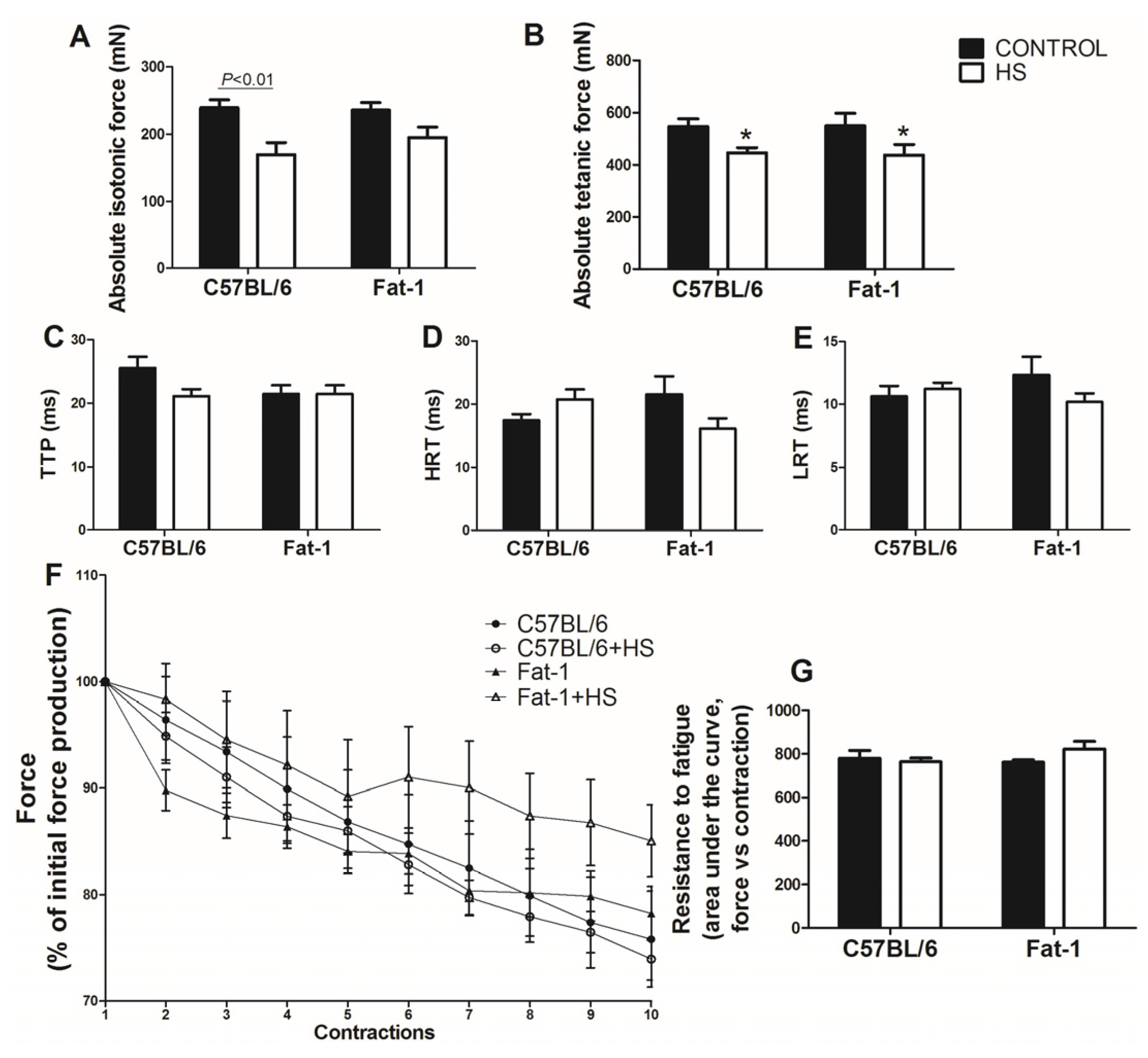
3.5. Analysis of Strength, Contractile Properties and Resistance to Fatigue in the Soleus Muscle
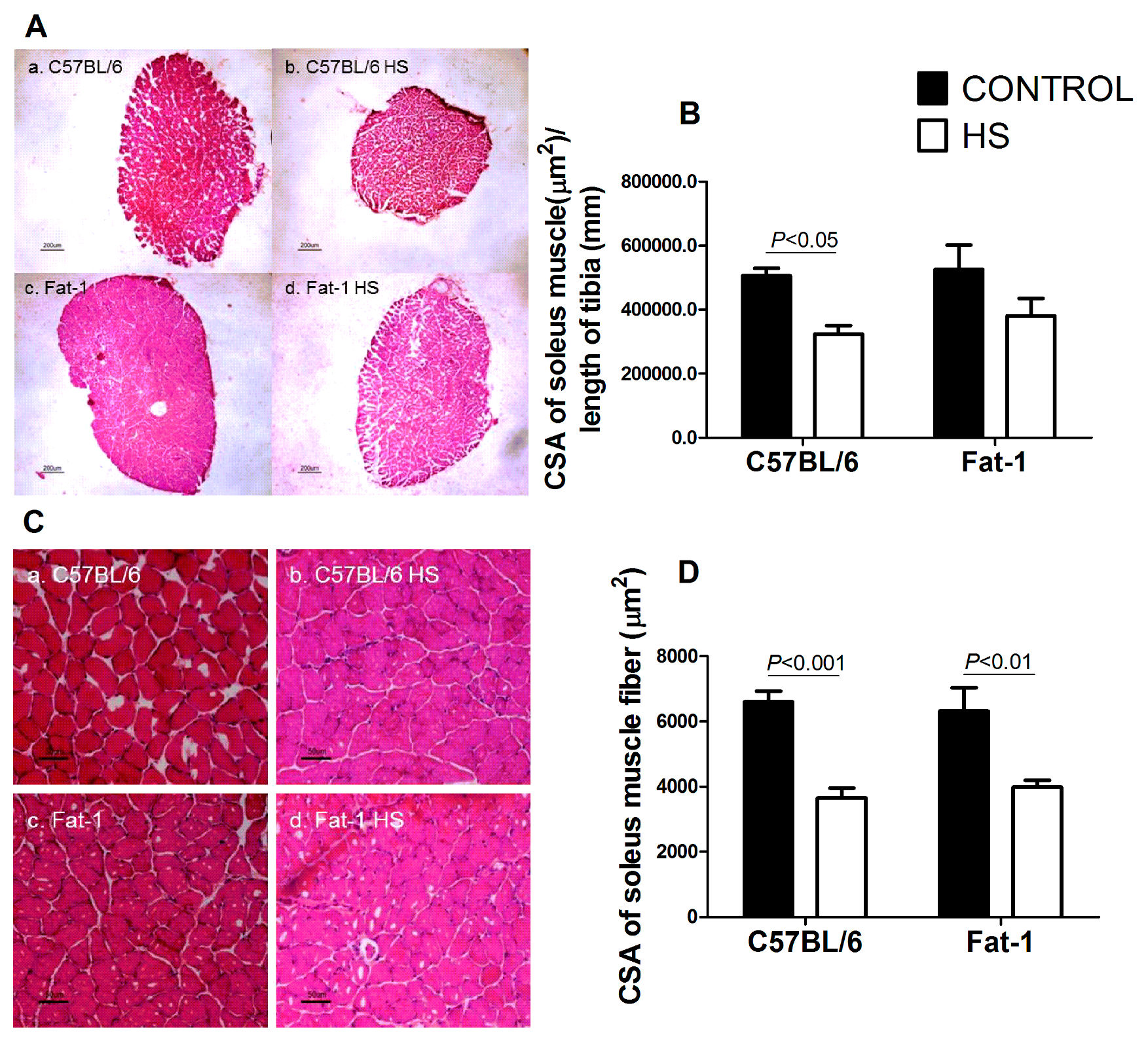
3.6. CSA of the Soleus Muscle and of Soleus Muscle Fibers
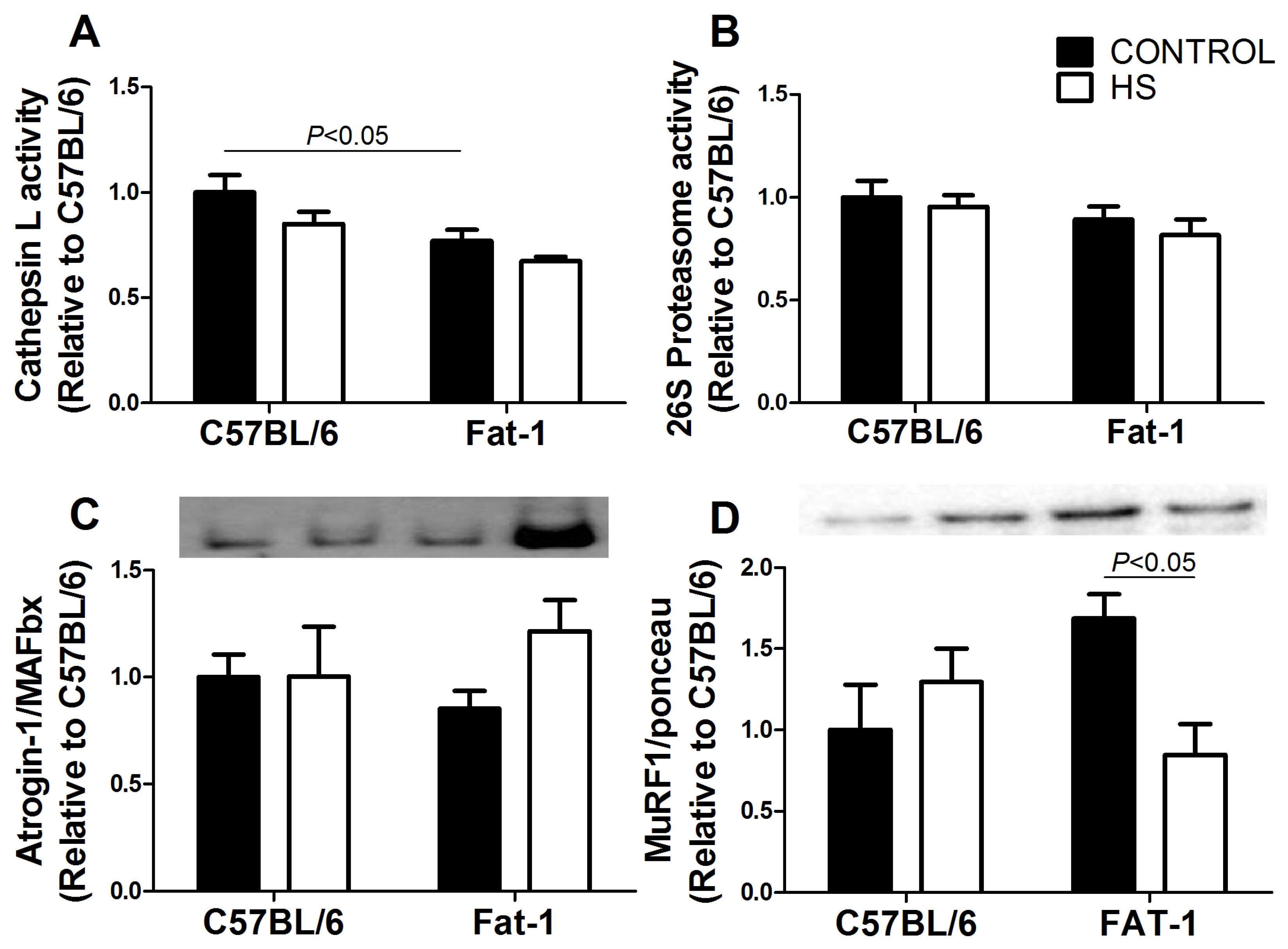
3.7. Protein Synthesis-Associated Signaling in Soleus Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marzuca-Nassr, G.N.; Vitzel, K.F.; De Sousa, L.G.; Murata, G.M.; Crisma, A.R.; Rodrigues Junior, C.F.; Abreu, P.; Torres, R.P.; Mancini-Filho, J.; Hirabara, S.M.; et al. Effects of high EPA and high DHA fish oils on changes in signaling associated with protein metabolism induced by hindlimb suspension in rats. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C. Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Greenhaff, P.L.; Phillips, S.M.; Bodine, S.C.; Adams, C.M.; Lang, C.H. Control of skeletal muscle atrophy in response to disuse: Clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E594–E604. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.; Feng, Z.; Shi, W.; Qu, L.; Li, Y.; Liu, J.; Long, J. Reloading functionally ameliorates disuse-induced muscle atrophy by reversing mitochondrial dysfunction, and similar benefits are gained by administering a combination of mitochondrial nutrients. Free Radic. Biol. Med. 2014, 69, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Winaga, H.; Sakurai, H.; Ohmichi, M.; Yoshimoto, T.; Ohmichi, Y.; Matsui, T.; Ushida, T.; Okada, T.; Sato, J. Treadmill running and static stretching improve long-lasting hyperalgesia, joint limitation, and muscle atrophy induced by cast immobilization in rats. Neurosci. Lett. 2013, 534, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Ishihara, A.; Murakami, S.; Yasuhara, T.; Kondo, H.; Mohri, S.; Takeda, I.; Roy, R.R. Protective effects of exercise preconditioning on hindlimb unloading-induced atrophy of rat soleus muscle. Acta Physiol. 2009, 197, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.K.; Dickinson, J.M.; Timmerman, K.L.; Drummond, M.J.; Reidy, P.T.; Fry, C.S.; Gundermann, D.M.; Rasmussen, B.B. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med. Sci. Sports Exerc. 2011, 43, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Baar, K.; Nader, G.; Bodine, S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006, 42, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Boonyarom, O.; Kozuka, N.; Matsuyama, K.; Murakami, S. Effect of electrical stimulation to prevent muscle atrophy on morphologic and histologic properties of hindlimb suspended rat hindlimb muscles. Am. J. Phys. Med. Rehabil. 2009, 88, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Dirks, M.L.; Hansen, D.; Van Assche, A.; Dendale, P.; Van Loon, L.J. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin. Sci. 2015, 128, 357–365. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Nabavi, S.M.; Micheletti, P.; Di Lorenzo, A.; Aquilani, R.; Nisoli, E.; Rondanelli, M.; Daglia, M. Creatine, L-carnitine, and omega3 polyunsaturated fatty acid supplementation from healthy to diseased skeletal muscle. Biomed. Res. Int. 2014, 2014, 613890. [Google Scholar] [CrossRef] [PubMed]
- De Campos-Ferraz, P.L.; Andrade, I.; das Neves, W.; Hangai, I.; Alves, C.R.; Lancha, A.H., Jr. An overview of amines as nutritional supplements to counteract cancer cachexia. J. Cachexia Sarcopenia Muscle 2014, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Magne, H.; Savary-Auzeloux, I.; Migne, C.; Peyron, M.A.; Combaret, L.; Remond, D.; Dardevet, D. Unilateral hindlimb casting induced a delayed generalized muscle atrophy during rehabilitation that is prevented by a whey or a high protein diet but not a free leucine-enriched diet. PLoS ONE 2013, 8, e70130. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; van Loon, L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Park, M.N.; Song, W.; Lee, Y.S. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl. Physiol. Nutr. Metab. 2010, 35, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.S.; Smith, H.J.; Drake, J.L.; Tisdale, M.J. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001, 61, 3604–3609. [Google Scholar] [PubMed]
- Gingras, A.A.; White, P.J.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K.; et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Kamolrat, T.; Gray, S.R.; Thivierge, M.C. Fish oil positively regulates anabolic signalling alongside an increase in whole-body gluconeogenesis in ageing skeletal muscle. Eur. J. Nutr. 2013, 52, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Wang, J.; Wu, L.; Kang, Z.B. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004, 427, 504. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.N.; Gladman, S.J.; Dyall, S.C.; Patel, U.; Virani, N.; Kang, J.X.; Priestley, J.V.; Michael-Titus, A.T. Transgenic mice with high endogenous omega-3 fatty acids are protected from spinal cord injury. Neurobiol. Dis. 2013, 51, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gladman, S.J.; Huang, W.; Lim, S.N.; Dyall, S.C.; Boddy, S.; Kang, J.X.; Knight, M.M.; Priestley, J.V.; Michael-Titus, A.T. Improved outcome after peripheral nerve injury in mice with increased levels of endogenous omega-3 polyunsaturated fatty acids. J. Neurosci. 2012, 32, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Thomason, D.B.; Herrick, R.E.; Surdyka, D.; Baldwin, K.M. Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J. Appl. Physiol. 1987, 63, 130–137. [Google Scholar] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MA, USA, 2005; Official method 996.06. [Google Scholar]
- American Oil Chemists’ Society (AOCS). Official Methods and Recommended Practices of the AOCS, 6th ed.; Firestone, D., Ed.; AOCS: Urbana, IL, USA, 2009. [Google Scholar]
- Abreu, P.; Pinheiro, C.H.; Vitzel, K.F.; Vasconcelos, D.A.; Torres, R.P.; Fortes, M.S.; Marzuca-Nassr, G.N.; Mancini-Filho, J.; Hirabara, S.M.; Curi, R. Contractile function recovery in severely injured gastrocnemius muscle of rats treated with either oleic or linoleic acid. Exp. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.; Hansen, B.F.; Urso, B.; Richter, E.A. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J. Appl. Physiol. 1996, 81, 1501–1509. [Google Scholar] [PubMed]
- Silveira, L.; Hirabara, S.M.; Alberici, L.C.; Lambertucci, R.H.; Peres, C.M.; Takahashi, H.K.; Pettri, A.; Alba-Loureiro, T.; Luchessi, A.D.; Cury-Boaventura, M.F.; et al. Effect of lipid infusion on metabolism and force of rat skeletal muscles during intense contractions. Cell. Physiol. Biochem. 2007, 20, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.H.; Vitzel, K.F.; Curi, R. Effect of N-acetylcysteine on markers of skeletal muscle injury after fatiguing contractile activity. Scand. J. Med. Sci. Sports 2012, 22, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.A.; Pinheiro, C.H.; Guimaraes-Ferreira, L.; Vitzel, K.F.; Vasconcelos, D.A.; Curi, R. Overload-induced skeletal muscle hypertrophy is not impaired in STZ-diabetic rats. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.H.; de Queiroz, J.C.; Guimaraes-Ferreira, L.; Vitzel, K.F.; Nachbar, R.T.; de Sousa, L.G.; de Souza-Jr, A.L.; Nunes, M.T.; Curi, R. Local injections of adipose-derived mesenchymal stem cells modulate inflammation and increase angiogenesis ameliorating the dystrophic phenotype in dystrophin-deficient skeletal muscle. Stem Cell Rev. 2012, 8, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.H.; Gerlinger-Romero, F.; Guimaraes-Ferreira, L.; de Souza-Jr, A.L.; Vitzel, K.F.; Nachbar, R.T.; Nunes, M.T.; Curi, R. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur J. Appl. Physiol. 2012, 112, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baar, K. Analysis of skeletal muscle hypertrophy in models of increased loading. Methods Mol. Biol. 2012, 798, 213–229. [Google Scholar] [PubMed]
- Jannig, P.R.; Moreira, J.B.; Bechara, L.R.; Bozi, L.H.; Bacurau, A.V.; Monteiro, A.W.; Dourado, P.M.; Wisloff, U.; Brum, P.C. Autophagy signaling in skeletal muscle of infarcted rats. PLoS ONE 2014, 9, e85820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchill, E.N.; Ferreira, J.C.; Brum, P.C.; Szweda, L.I.; Mochly-Rosen, D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc. Res. 2010, 85, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.F.; Bacurau, A.V.; Moreira, J.B.; Paixao, N.A.; Campos, J.C.; Ferreira, J.C.; Leal, M.L.; Negrao, C.E.; Moriscot, A.S.; Wisloff, U.; et al. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS ONE 2012, 7, e41701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fortes, M.A.; Marzuca-Nassr, G.N.; Vitzel, K.F.; da Justa Pinheiro, C.H.; Newsholme, P.; Curi, R. Housekeeping proteins: How useful are they in skeletal muscle diabetes studies and muscle hypertrophy models? Anal. Biochem. 2016, 504, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calvo, I.; Ocon, B.; Martinez-Moya, P.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Gilda, J.E.; Gomes, A.V. Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007, 102, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Li, Y.; Hannon, K.; Watkins, B.A. Hind limb suspension and long-chain omega-3 PUFA increase mRNA endocannabinoid system levels in skeletal muscle. J. Nutr. Biochem. 2012, 23, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.A.; Lang, C.H.; Zhang, Y.; Paul, E.M.; Laufenberg, L.J.; Lewis, G.S.; Donahue, H.J. Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J. Bone Miner. Res. 2014, 29, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Belzung, F.; Raclot, T.; Groscolas, R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am. J. Physiol. 1993, 264, R1111–R1118. [Google Scholar] [PubMed]
- Baillie, R.A.; Takada, R.; Nakamura, M.; Clarke, S.D. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 351–356. [Google Scholar] [CrossRef]
- Parra, D.; Ramel, A.; Bandarra, N.; Kiely, M.; Martinez, J.A.; Thorsdottir, I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008, 51, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Bederman, I.R.; Chandramouli, V.; Sandlers, Y.; Henderson, L.; Cabrera, M.E. Time course of hepatic gluconeogenesis during hindlimb suspension unloading. Exp. Physiol. 2013, 98, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J. Appl. Physiol. 2000, 89, 823–839. [Google Scholar] [PubMed]
- Maki, T.; Yamamoto, D.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; Kaji, H.; Chihara, K.; Okimura, Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr. Res. 2012, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Soukup, T.; Zacharova, G.; Smerdu, V. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem. 2002, 104, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.M.; Harrison, B.C.; Young, M.H.; Stodieck, L.S.; Ferguson, V.L. Longitudinal characterization of functional, morphologic, and biochemical adaptations in mouse skeletal muscle with hindlimb suspension. Muscle Nerve 2013, 48, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Lalia, A.Z.; Dasari, S.; Pallauf, M.; Fitch, M.; Hellerstein, M.K.; Lanza, I.R. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 2015, 14, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Inoue, T.; Katakura, M.; Hossain, S.; Mamun, A.A.; Matsuzaki, K.; Arai, H.; Shido, O. Differential effects of docoosahexaenoic and arachidonic acid on fatty acid composition and myosin heavy chain-related genes of slow- and fast-twitch skeletal muscle tissues. Mol. Cell. Biochem. 2016, 415, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Sawano, S.; Mashima, D.; Ichitsubo, R.; Nakamura, M.; Tatsumi, R.; Ikeuchi, Y.; Mizunoya, W. Mouse soleus (slow) muscle shows greater intramyocellular lipid droplet accumulation than EDL (fast) muscle: fiber type-specific analysis. J. Muscle Res. Cell Motil. 2017. [Google Scholar] [CrossRef]
- Feng, H.Z.; Chen, X.; Malek, M.H.; Jin, J.P. Slow recovery of the impaired fatigue resistance in postunloading mouse soleus muscle corresponding to decreased mitochondrial function and a compensatory increase in type I slow fibers. Am. J. Physiol. Cell Physiol. 2016, 310, C27–C40. [Google Scholar] [PubMed]
- Ohira, T.; Kawano, F.; Ohira, T.; Goto, K.; Ohira, Y. Responses of skeletal muscles to gravitational unloading and/or reloading. J. Physiol. Sci. 2015, 65, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.A.; Paglialunga, S.; Gerling, C.; Whitfield, J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.; Spriet, L.L.; Holloway, G.P. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 2014, 592, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Peoples, G.E.; McLennan, P.L. Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. Br. J. Nutr. 2010, 104, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.; Peoples, G.E.; McLennan, P.L. Muscle fatigue resistance in the rat hindlimb in vivo from low dietary intakes of tuna fish oil that selectively increase phospholipid n-3 docosahexaenoic acid according to muscle fibre type. Br. J. Nutr. 2015, 114, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Zergeroglu, A.M.; Demirel, H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; West, D.W.; Marcotte, G.; Marshall, A.G.; De Sousa, L.G.; Baar, K.; Bodine, S.C. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging 2016, 8, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Andrianjafiniony, T.; Dupre-Aucouturier, S.; Letexier, D.; Couchoux, H.; Desplanches, D. Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. Am. J. Physiol. Cell Physiol. 2010, 299, C307–C315. [Google Scholar] [CrossRef] [PubMed]
| C57BL/6 | C57BL/6 + HS | Fat-1 | Fat-1 + HS | |
|---|---|---|---|---|
| Body weight | ||||
| Initial body weight (g) | 23 ± 0.6 | 23 ± 0.5 | 22 ± 0.4 | 24 ± 0.7 |
| Increase or decrease of body mass after 14 days (g) | 1.6 ± 0.3 | −0.7 ± 0.3 b | 1.3 ± 0.4 | −1.7 ± 0.8 c |
| Soleus muscle | ||||
| Wet weight (mg/mm tibia length) | 0.456 ± 0.019 | 0.263 ± 0.010 c | 0.435 ± 0.013 | 0.319 ± 0.021 c,x |
| Percent loss due to HS in wet weight | 42% | 27% | ||
| Dry weight (mg/mm tibia length) | 0.149 ± 0.010 | 0.112 ± 0.010 a | 0.151 ± 0.017 | 0.137 ± 0.012 |
| Percent loss due to HS in dry weight | 25% | 9% | ||
| Gastrocnemius muscle | ||||
| Wet weight (mg/mm tibia length) | 6.945 ± 0.168 | 5.334 ± 0.144 c | 6.608 ± 0.213 | 5.473 ± 0.188 c |
| Percent loss due to HS in wet weight | 23% | 17% | ||
| Dry weight (mg/mm tibia length) | 1.683 ± 0.046 | 1.320 ± 0.046 c | 1.649 ± 0.056 | 1.355 ± 0.063 b |
| Percent loss due to HS in dry weight | 22% | 18% | ||
| Plantaris muscle | ||||
| Wet weight (mg/mm tibia length) | 0.892 ± 0.019 | 0.697 ± 0.010 c | 0.914 ± 0.013 | 0.784 ± 0.021 c,y |
| Percent loss due to HS in wet weight | 22% | 14% | ||
| Dry weight (mg/mm tibia length) | 0.218 ± 0.009 | 0.171 ± 0.006 b | 0.232 ± 0.012 | 0.187 ± 0.010 b |
| Percent loss due to HS in dry weight | 22% | 19% | ||
| Tibialis anterior muscle | ||||
| Wet weight (mg/mm tibia length) | 2.237 ± 0.074 | 1.860 ± 0.063 c | 2.369 ± 0.098 | 1.949 ± 0.071 b |
| Percent loss due to HS in wet weight | 17% | 18% | ||
| Dry weight (mg/mm tibia length) | 0.635 ± 0.015 | 0.565 ± 0.025 | 0.645 ± 0.034 | 0.536 ± 0.025 a |
| Percent loss due to HS in dry weight | 11% | 17% | ||
| EDL muscle | ||||
| Wet weight (mg/mm tibia length) | 0.478 ± 0.020 | 0.434 ± 0.021 | 0.544 ± 0.015 x | 0.469 ± 0.017 a |
| Percent loss due to HS in wet weight | 9% | 14% | ||
| Dry weight (mg/mm tibia length) | 0.141 ± 0.009 | 0.122 ± 0.009 | 0.137 ± 0.007 | 0.128 ± 0.008 |
| Percent loss due to HS in dry weight | 14% | 7% | ||
| Subcutaneous fat mass | ||||
| Wet weight (mg/cm L) | 22.49 ± 2.40 | 22.55 ± 2.98 | 21.03 ± 1.30 | 18.51 ± 2.46 |
| Percent loss due to HS in wet weight | 0% | 12% | ||
| Epididymal fat mass | ||||
| Wet weight (mg/cm L) | 26.23 ± 1.56 | 15.37 ± 0.81 c | 19.82 ± 1.30 y | 11.16 ± 1.57 c |
| Percent loss due to HS in wet weight | 41% | 44% | ||
| Retroperitoneal fat mass | ||||
| Wet weight (mg/cm L) | 6.80 ± 0.93 | 7.40 ± 0.72 | 6.72 ± 0.46 | 4.56 ± 0.54 x |
| Percent loss due to HS in wet weight | 0% | 32% | ||
| Mesenteric fat mass | ||||
| Wet weight (mg/cm L) | 17.21 ± 2.96 | 13.35 ± 2.55 | 15.48 ± 2.57 | 12.90 ± 3.29 |
| Percent loss due to HS in wet weight | 22% | 17% | ||
| Soleus total protein content | ||||
| mg/g fresh tissue | 34.33 ± 7.84 | 43.00 ± 9.72 | 62.47 ± 7.16 x | 59.67 ± 1.22 |
| Fatty Acid | Name | C57BL/6 | C57BL/6 + HS | Fat-1 | Fat-1 + HS |
|---|---|---|---|---|---|
| 14:0 | Myristic | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 16:0 | Palmitic | 0.29 ± 0.04 | 0.28 ± 0.02 | 0.23 ± 0.10 | 0.30 ± 0.03 |
| 16:1 (ω-7) | Hexadecenoic | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 c |
| 17:0 | Margaric | - | - | - | - |
| 18:0 | Stearic | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.11 ± 0.05 | 0.13 ± 0.01 |
| 18:1 (ω-9) | Oleic | 0.18 ± 0.06 | 0.15 ± 0.04 | 0.13 ± 0.07 | 0.18 ± 0.04 |
| 18:1 (ω-11) | Vaccenic | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| 18:2 (ω-6) | Linoleic | 0.25 ± 0.07 | 0.21 ± 0.03 | 0.21 ± 0.09 | 0.24 ± 0.04 |
| 20:0 | Eicosanoic | - | - | - | - |
| 20:1 (ω-9) | Eicosenoic | - | - | - | - |
| 18:3 (ω-6) | γ-Linolenic | - | - | - | - |
| 18:3 (ω-3) | α-Linolenic | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| 22:0 | Docosanoic | - | - | - | - |
| 20:2 (ω-6) | Eicosadienoic | 0.01 ± 0.00 | - | - | - |
| 20:3 (ω-9) | Eicosatrienoic | 0.01 ± 0.00 | 0.01 ± 0.00 | - | - |
| 20:4 (ω-6) | Arachidonic | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.02 ± 0.01 z | 0.01 ± 0.00 z |
| 22:2 (ω-6) | Docosadienoic | - | - | - | — |
| 20:5 (ω-3) | Eicosapentaenoic | - | - | 0.01 ± 0.00 | 0.02 ± 0.01 |
| 22:4 (ω-3) | Docosatetraenoic | 0.02 ± 0.00 | 0.02 ± 0.00 | - | - |
| 22:5 (ω-6) | Docosapentaenoic | 0.02 ± 0.00 | 0.02 ± 0.00 | - | - |
| 22:5 (ω-3) | Docosapentaenoic | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.02 z | 0.06 ± 0.01 z |
| 22:6 (ω-3) | Docosahexaenoic | 0.13 ± 0.02 | 0.15 ± 0.04 | 0.15 ± 0.07 | 0.21 ± 0.03 |
| Total | Saturated | 0.44 ± 0.06 | 0.42 ± 0.03 | 0.35 ± 0.16 | 0.44 ± 0.04 |
| Monounsaturated | 0.25 ± 0.08 | 0.23 ± 0.06 | 0.18 ± 0.09 | 0.27 ± 0.06 | |
| Polyunsaturated | 0.59 ± 0.11 | 0.57 ± 0.08 | 0.45 ± 0.20 | 0.56 ± 0.06 | |
| ω-3 | 0.16 ± 0.03 | 0.19 ± 0.04 | 0.22 ± 0.10 | 0.31 ± 0.04 a,y | |
| ω-6 | 0.42 ± 0.08 | 0.39 ± 0.04 | 0.23 ± 0.10 z | 0.25 ± 0.04 y | |
| ω-6/ω-3 ratio | 2.62±0.39 | 2.11 ± 0.27 b | 1.14 ± 0.27 z | 0.79 ± 0.11 y | |
| Total fat % | 1.37±0.25 | 1.32 ± 0.17 | 1.25 ± 0.10 | 1.37 ± 0.16 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzuca-Nassr, G.N.; Murata, G.M.; Martins, A.R.; Vitzel, K.F.; Crisma, A.R.; Torres, R.P.; Mancini-Filho, J.; Kang, J.X.; Curi, R. Balanced Diet-Fed Fat-1 Transgenic Mice Exhibit Lower Hindlimb Suspension-Induced Soleus Muscle Atrophy. Nutrients 2017, 9, 1100. https://doi.org/10.3390/nu9101100
Marzuca-Nassr GN, Murata GM, Martins AR, Vitzel KF, Crisma AR, Torres RP, Mancini-Filho J, Kang JX, Curi R. Balanced Diet-Fed Fat-1 Transgenic Mice Exhibit Lower Hindlimb Suspension-Induced Soleus Muscle Atrophy. Nutrients. 2017; 9(10):1100. https://doi.org/10.3390/nu9101100
Chicago/Turabian StyleMarzuca-Nassr, Gabriel Nasri, Gilson Masahiro Murata, Amanda Roque Martins, Kaio Fernando Vitzel, Amanda Rabello Crisma, Rosângela Pavan Torres, Jorge Mancini-Filho, Jing Xuan Kang, and Rui Curi. 2017. "Balanced Diet-Fed Fat-1 Transgenic Mice Exhibit Lower Hindlimb Suspension-Induced Soleus Muscle Atrophy" Nutrients 9, no. 10: 1100. https://doi.org/10.3390/nu9101100





